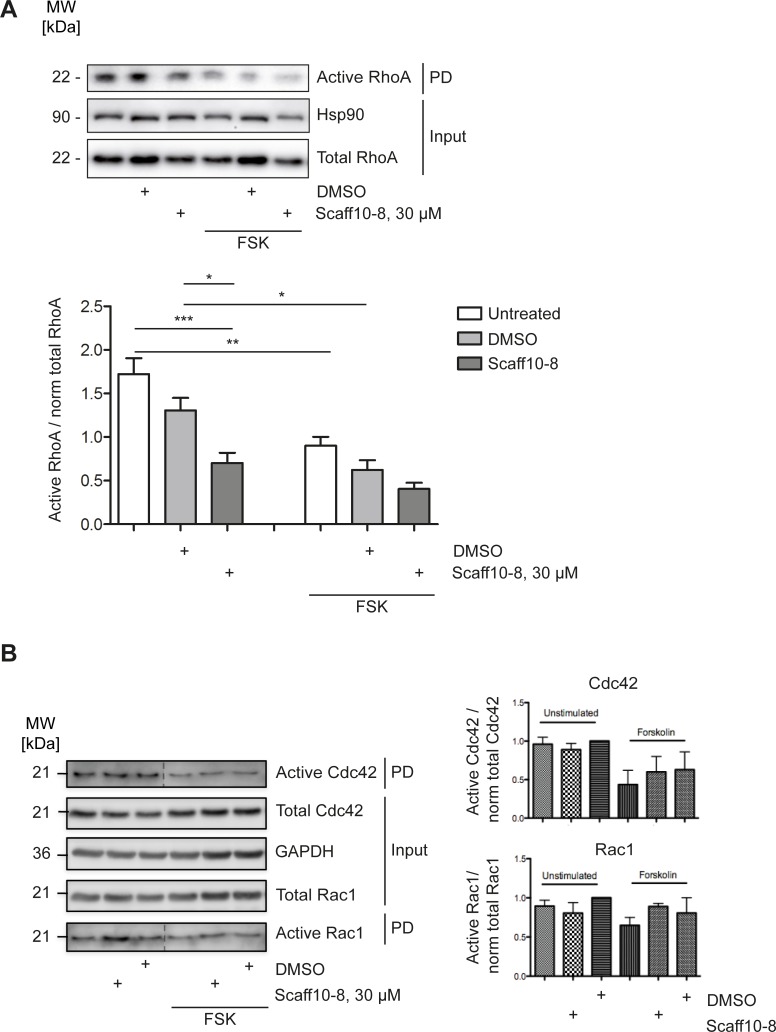
Fig 10. Scaff10-8 inhibits activation of RhoA in primary IMCD cells.
IMCD cells were incubated with Scaff10-8 (30 μM, 1 h). DMSO (1%), the solvent of Scaff10-8, served as a control. The cells were lysed, (A) active RhoA was precipitated with the RhoA binding domain of Rhotekin coupled to sepharose beads, (B) active Cdc42 and Rac1 were precipitated with with GST fused to the (p21) binding domain (PBD) of p21 activated kinase 1 protein (PAK-1) coupled to sepharose beads. Inputs and pulldown fractions were separated by SDS-PAGE. Hsp90 or GAPDH were used as loading controls. Representative Western blots from 3–5 independent experiments are shown. Signals were semiquantitatively analyzed by densitometry. The amount of active RhoA was related to normalized RhoA (total RhoA to Hsp90 (Input)). Accordingly, active Cdc42 and Rac1 were related to normalized Cdc42 and Rac1, respectively (total RhoA to GAPDH (Input)). PD, Pulldown. n = 3–5. Statistically significant differences were determined using one-way ANOVA with posthoc Bonferroni. Mean ± SEM is plotted. *, p ≤ 0.05; **, p ≤ 0.01; *** p ≤ 0.001.