Abstract
Human embryonic stem cell (hESC)-derived hematopoietic stem/progenitor cells hold tremendous potential as alternative cell sources for the treatment of various hematological diseases, drug discovery and toxicological screening. However, limited number of hematopoietic stem/progenitor cells generated from the differentiation of hESCs hinders their downstream applications. Here, we show that aryl hydrocarbon receptor antagonist StemRegenin 1 (SR1) selectively promotes expansion of hESC-derived lin-CD34+ hematopoietic progenitors in a concentration-dependent manner. The colony-forming cell (CFC) activity was found to be enriched in the CD34+ cells that were expanded with SR1; however, these cells have less colony-forming activity as compared to unexpanded cells (1,338 vs. 7 of CD34+ cells to form 1 colony, respectively). Interestingly, SR1 showed a bipotential effect on the proliferation of CD34 negative population, that is low dose of SR1 (1 µM) enhanced cell proliferation, whereas it was repressed at higher doses (>5 µM). In summary, our results suggest that SR1 has the potential to facilitate expansion of hESC-derived lin-CD34+ hematopoietic progenitors, which further retain the potential to form multilineage hematopoietic colonies.
Keywords: SR1, Hematopoietic progenitor, Human embryonic stem cell, CD43
Introduction
A member of the family of basic helix-loop-helix transcription factors, aryl hydrocarbon receptor (AHR) is well known for regulating the function of xenobiotic-metabolizing enzymes, and the toxicity and carcinogenic properties of several compounds[1]. AHR has also been proven to play an important role in the regulation of pluripotency and stemness of hematopoietic stem cells (HSCs). Inhibition of AHR by StemRegenin 1 (SR1) has been shown to lead to a 50-fold increase in cells expressing CD34 and a 17-fold increase in cells that retain the ability to engraft immunodeficient mice[2].
Conventional sources such as umbilical cord blood, bone marrow, and peripheral blood contain very limited number of HSCs. Additionally, cumbersome tissue extraction procedures, risk of blood-borne pathogen contamination and MHC mismatch issues are the major problems associated with the shortage of available HSCs required to meet the therapeutic demand at the blood banks and clinics. Alternatively, human embryonic stem cells (hESCs) have the potential to form massive numbers of HSCs when cultured under hematopoietic differentiation inducing conditions.
Hematopoietic development from hESCs using mouse bone marrow stromal cell line, OP9, as feeder starts with the formation of primitive hematovascular mesodermal precursors (HVMPs), defined phenotypically as KDRbrightAPLNR+PDGFRαlow/-. A transient population of VE - cadherin+CD73-CD235a/CD43- hemogenic endothelial progenitors (HEPs) gradually emerge from HVMP (day 4) which subsequently undergoes endothelial-to-hematopoietic transition[3]. A more committed CD43+CD235a/41a- multipotent hematopoietic progenitors (MPs) with hematopoietic colony-forming ability can be obtained from hESC/OP9 cocultures after 8 days of differentiation[4]. Although MPs can be expanded for additional 8 days in culture when supplemented with hematopoietic cytokines, they gradually lose CD34 expression and colony forming ability[5].
Multipotent hematopoietic progenitors are developmental intermediate between hematopoietic stem cells (HSCs) and mature hematopoietic cells of all lineages (i.e., erythro-megakaryocytic, myeloid, and lymphoid). They have immunophenotypic profile similar to HSCs such as CD34 expression and lacking of lineage markers (CD11b, CD14, CD2, CD3, CD7, CD19, CD38, CD41a, CD45RA, HLA-DR, and CD235a)[4]. Currently, therapeutic potential of blood cell products obtained from hESC-derived HSCs/MPs have been intensely investigated. Specifically, hESC-derived natural killer (NK) cells demonstrated cellular cytotoxic activity and effectively eradicated human tumor cells in vivo[6]. The Food and Drug Administration (USFDA) recently approved a chimeric antigen receptor (CAR) T lymphocytes for the treatment of acute lymphoblastic leukemia and a model of CAR T lymphocyte generated from induced pluripotent stem cells for cancer therapy has been reported[7]. Hence, in vitro expansion of hESC-derived MPs to achieve sufficient number of cells for cancer immunotherapy and other medical applications is a prerequisite.
Aim of the present study was to evaluate effects of SR1 in the expansion of MPs derived from hESC/OP9 co-culture. Our data would highlight an effective strategy for the in-vitro expansion of hematopoietic progenitors derived from hESCs that would provide an unlimited source of cells for devising cellular therapies for various hematological disorders and malignancies.
Materials and Methods
Maintenance of WA01 and their differentiation into hematopoietic lineage on OP9 feeders
The human embryonic stem cell (hESC) line WA01 was obtained from WiCell and maintained in an undifferentiated state on irradiated mouse embryonic fibroblasts (MEFs). OP9 stromal cells were procured from ATCC and were maintained on gelatin-coated 10 cm dishes (BD Biosciences) in the OP9 growth medium consisting of 20% FBS (Gibco) in α-MEM medium (Invitrogen). Hematopoietic differentiation of WA01 cells on OP9 feeders was performed as previously described[4,8] in differentiation medium containing α-MEM basal medium supplemented with 10% FBS (HyClone), 100 µM monothioglycerol (MTG; Sigma Aldrich) and ascorbic acid (50 µg/ml) (Sigma Aldrich). MPs were derived on day 8 of WA01/OP9 co-culture.
Isolation of WA01-derived lin-CD43+CD235a/41a- MPs
Cells were obtained by digesting the differentiated WA01/OP9 co-cultures with collagenase IV (1 mg/ml) (Invitrogen) followed by treatment with 0.05% trypsin-EDTA (Invitrogen) for 15 minutes at 37°C. Single cells were obtained by passing the digested cells through a 100-µM cell strainer (BD Biosciences) and counted. Cells were labeled with CD43 monoclonal antibody (clone 1G10) for CD43+ hematopoietic cell enrichment using magnetic-activated cell separation columns according to manufacturer’s intruction (Miltenyi Biotec). Subsequently, CD43-enriched cells were stained with CD34, CD235a, and CD41a monoclonal antibodies, and lin-CD34+CD43+CD235a/CD41a- MP cells were isolated by fluorescence-activated cell sorting (FACSAria, BD Biosciences). All monoclonal antibodies were from BD Biosciences.
Flow cytometric analysis of expanded MPs
Expanded MPs were stained with CD34 and CD43 monoclonal antibodies for flow cytometric analysis. Isotype-matched controls were used to set threshold for background. Data was acquired on a FACS Canto flow cytometer (BD Biosciences). 7-aminoactinomycin D (7AAD) was used to discriminate live cells from dead cells, and the stained live single cells were analyzed on FlowJo (Tree Star, Inc.).
Hematopoietic colony-forming unit (CFU) assay
Single cells were plated at a density of 200 cells/35-mm dish in MethoCult GF H4435 (StemCell Technologies). Colonies were scored after 14 days according to their morphology as granulocyte (G), macrophage (M), granulocyte/macrophage (GM), and multilineage colonies containing erythroid and non-erythroid cells (GEMM) as previously described[4,9].
Cell proliferation assay
lin-CD34+CD43+CD235a/CD41a- MPs were plated in duplicate in 96-well plates containing 4x103 cells/well. Cells were cultured in serum-free medium containing 10%BIT (StemCell Technologies), 100 µM 2-mercaptoethanol, and ExCyte (Millipore) in IMDM supplemented with 10 ng/ml IL3, and 50 ng/ml IL6 and SCF. SR1 was added to the cultures at concentrations ranging from 1, 5 and 10 µM (Cayman Chemical). Viable cell count was determined using trypan blue (Gibco).
RNA-Seq analysis
To visualize the comparative gene expression levels of genes expressed in WA01, HVMPs, HEs, and MPs, a heat map was constructed using MultiExperiment Viewer v4.2 (http://www.tm4.org). RNA-seq data was obtained from NCBI GEO DataSets (acession number: GSE39661). The gene expression levels were estimated in transcripts per million (tpm) as described earlier[3]. TPM for MP was averaged from D8 CD43+CD235a- and D8 CD43+CD235a+ subset.
Statistical analysis
Data obtained from three experiments were reported as mean ± SEM. Significance of the data was determined by ANOVA followed by Bonferroni post hoc test. Pearson correlation between CD34 expression and colony-forming cell (CFC) was determined. p value < 0.05 was considered statistically significant. All the graphs and statistics were done on Prism software (GraphPad).
Results
Expression profile of aryl hydrocarbon receptor and its associated genes are upregulated in hematopoietic progenitors derived from differentiated pluripotent stem cells
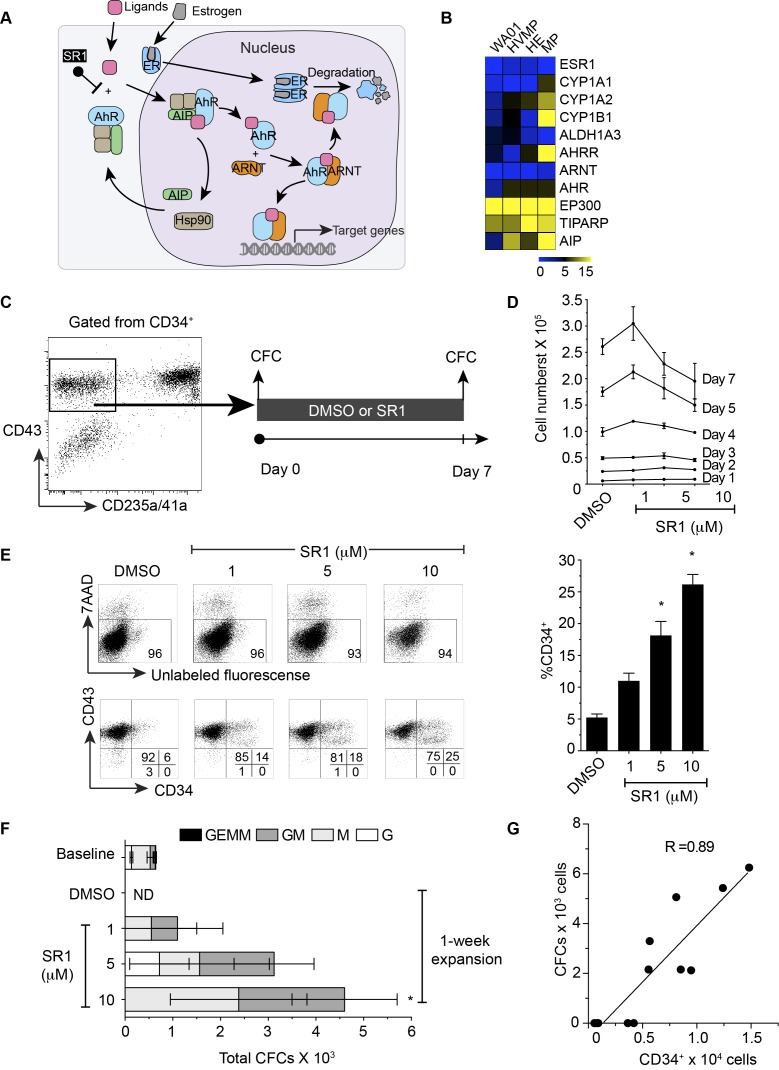
In this study, we have examined the gene expression profile of WA01 and its differentiated hematopoietic derivatives such as HVMPs, Hemogenic endothelia (HEs), and MPs, which was previously generated by our group[3]. It was revealed that several AHR-associated genes (Figure 1A) namely CYP1A1, CYP1A2, CYP1B1, AHRR, ARNT, AHR, EP300, TIPARP, and AIP were upregulated as cells acquired CD43 expression, suggesting that AHR signaling is activated in committed hematopoietic cells (Figure 1B). This information prompted us to further evaluate whether inhibition of AHR activity will lead to the sustained maintenance of naive multipotent phenotype and functionality of MPs.
Figure 1.
Aryl hydrocarbon signaling and its effects on multipotent hematopoietic progenitor cells. (A) Schematic diagram showing current mechanisms of AHR regulation. (B) Heat map showing the expression of genes associated with AHR signaling and estrogen receptor in WA01, HVMP, HE, and MP. (C) Schematic diagram showing the experimental outline. FACS-sorted live CD235a/41a-CD34+CD43+ cells from day 8 WA01/OP9 coculture shown in the gate were cultured in the presence or absence of SR1 for 7 days. The sorted cells (day 0) and expanded cells (day 7) were plated in methylcellulose for the colony-forming cell (CFC) assay. (D) Cross-sectional presentation of total viable cell numbers on a day-to-day basis. * indicates significant difference compared with DMSO. (E) Representative flow cytometric dot plots showing the effect of SR1 on cell viability after 7-day expansion using 7AAD (upper panel) and CD43 vs CD34 expression on the expanded cells (lower panel). Right bar graph summarizes a percentage of CD34 expression shown in the dot plots. (F) Absolute number of colony-forming cells in WA01-derived MPs (baseline, day 0), expanded cells with DMSO only, and expanded cells with 1, 5 and 10 µM SR1 (G) Correlation between colony-forming cells and CD34 expression. Error bars represent SEM from 3 experiments. * indicates p < 0.05 compared to DMSO. HVMP = hematovascular mesodermal precursor, HE = hemogenic endothelium, MP = multipotent hematopoietic progenitor, ER = estrogen receptor, AHR = aryl hydrocarbon receptor, AIP = AHR interacting protein, ARNT = AHR nuclear translocator, Hsp90 = heat shock protein 90, SR1 = StemRegenin 1, ND = not detectable
SR1 promotes expansion of MPs without deteriorating cell viability
To generate MPs, OP9 stromal cells were used to induce hematopoietic differentiation in WA01. The experimental outline for the generation of MPs and examination of their colony-forming activities are shown in Figure 1C. On day 8 of WA01/OP9 co-culture, MPs are found to be enriched within CD43+glycophorin A- (CD235a) CD41a- population[5]. Approximately 2-5x104 of MPs can be obtained from a single 10 cm dish containing hESC/OP9 co-cultures. Effects of SR1 at concentrations >1 µM (high dose) has been shown anti-proliferative in cynomolgus, rhesus, and dog[2], however, such effects of SR1 on human CD34+ cells remains poorly understood. Hereby, we have evaluated the effects of high doses SR1 on MPs grown under serum free conditions through viable cell count analysis, flow cytometry and CFU assays. We limited our experiments to a short 7-day assay because WA01-derived MPs slowly lose CD34 expressions and almost completely disappear within the first week[5]. Our data showed that after 7 days of expansion cultures, SR1 at 1 µM slightly increased total cell expansion of MPs (p>0.5), whereas at 10 µM doses it markedly inhibited expansion (Figure 1D). Interestingly, flow cytometric analyses using live/dead staining marker (7AAD) showed that a reduction in expansion resulted from anti-proliferative effects of SR1 at high dose, not the cell death (Figure 1E).
SR1 enhances CD34 expression and its hematopoietic progenitor activity in a dose-dependent manner
At the late stage of MP maturation, mature cells retain CD43, but lose CFU activity[5]. Therefore, we examined the expression of another hematopoietic progenitor cell marker CD34 and its colony-forming activity through culturing MPs for 7-days in the absence or presence of SR1 (Figure 1E). We found that SR1 maintained CD34 expression on MPs in a dose-dependent fashion, but had no effect on the CD43 expression (Figure 1E). To evaluate the colony-forming ability of CD34+ at different stages of culture, 200 cells of sorted CD34+ (unexpanded) were plated in methylcellulose medium containing hematopoietic cytokines as a baseline control (baseline). After 7-day expansion, 200 cells from each condition (DMSO, 1, 5, 10 µM of SR1) were plated in the same medium as baseline control. We could not detect any hematopoietic colonies from the expanded cultures grown without SR1 (Figure 1F). We noticed that colonies obtained from MPs after expansion were slightly smaller than those colonies obtained from unexpanded MPs indicating a gradual decrease in their proliferative potential. We hypothesized that CFCs might be enriched within the expansion cultures of CD34+ population. Thus, we performed correlation analysis on CD34 and CD43 vs. CFCs. We found a strong positive correlation between CD34 vs. CFCs (R=0.89, p=0.0001) (Figure 1G), but not CD43. Estimated frequency of colony forming cell in CD34+ population from expanded cultures was 1,338 cells to form 1 colony.
Discussion
The abilities of HSCs to self-renew and differentiate into mature cells highlight their potential as a valuable source for cell replacement therapy for various hematological diseases and high-throughput platform for drug discovery and toxicological screening. Limited availability of HSCs in the conventional donor cell sources, and the difficulty of ex vivo expansion of HSCs that retain all their functional attributes, pose as the major hurdles in the clinical applications of HSCs in treating various blood disorders and malignancies. With the discovery of hESC[10], cells with HSC-like phenotype can be generated unlimitedly in a dish[11]. hESC-derived cells as an attractive complementary cell source for therapeutic interventions, disease modeling and drug-screening. Although several groups describe efficient methods to generate de novo HSC-like cells from hESCs (reviewed earlier[12]), yield of cells remains one of the major hindrances in its downstream applications. Ectopic expression of HOXB4 and BMI1 transcription factors has been demonstrated to promote ex vivo expansion of HSCs[13,14]; however, use of virus-mediated gene transfer is the major drawback in the clinical translation of these findings. Therefore, optimization of in vitro expansion conditions to generate sufficient quantities of hESC-derived HSC-like cells is the need of the hour.
SR1 has been widely used in several protocols to promote HSC and hematopoietic cell expansión[2,15,16] and is currently under phase I/II clinical trial in patients with hematologic malignancies[17]. We found that several AHR target genes are upregulated when cells commit to hematopoietic lineage (lin-CD34+CD43+). We demonstrated that SR1 favored expansion of progenitor cells regardless of the concentration used, but its effect on mature cells was found to be bipotential. Consistent to the report by Boitano, et al[2], SR1 at low dose (1 µM) facilitated mature cell proliferation, whereas high doses (>1 µM) suppressed total cell numbers. Flow cytometric analyses using 7AAD showed that SR1 did not cause cell death when used at high doses confirming the anti-proliferative effect is most likely. Although the effect of SR1 is mediated through direct binding and inhibition of the AHR[2], the precise mechanism of its bipotential effect on mature cells remains undescribed.
It is possible that SR1 at high dose has off target effects that initiate secondary signaling pathway. Functional study on the expanded cells showed that the effect on hematopoietic progenitor activity is concentration-dependent suggesting the benefit of high concentration of SR1 on MP expansion. Additionally, a strong positive correlation between CD34 vs. CFCs (R=0.89, p=0.0001) suggested an advantage of CD34 over CD43 as a marker for colony forming activity in the expanded cells. Notably, the numbers of colony-forming cells within the expanded CD34+ population is much lower than unexpanded cells indicating that even though SR1 favors expansion of CD34+ progenitor cells, some cells might undergo further differentiation.
Conclusion
In conclusion, our data demonstrates that high doses SR1 (>1 µM) selectively expand MP derived from WA01 (5-fold increase when used at 10 µM) without having deteriorating effects on cell viability. Mechanistic study of the bipotential effect of SR1 on MPs would provide insight to devise a novel approach for expansion of embryonic stem cell-derived blood products.
Glossary
Abbreviations
- AHR:
Aryl hydrocarbon receptor
- hESC:
human embryonic stem cell
- SR1:
StemRegenin 1
- CFC:
Colony-forming cell
- HE:
Hemogenic endothelium
- HEP:
Hemogenic endothelial progenitor
- HVMP:
Hematovascular mesodermal precursor
- MP:
Multipotent hematopoietic progenitors
- FBS:
Fetal bovine serum
- MTG:
Monothioglycerol
- G:
Granulocyte
- M:
Macrophage
- GM:
Granulocyte/Macrophage
- GEMM:
Granulocyte/Erythrocyte/Macrophage
Potential Conflicts of Interests
None
Funding
This work was supported by funds from the Department of Pharmacology, Faculty of Science, Mahidol University (A34/2557, grant to KS) and The Central Instrument Facility (CIF), Mahidol University (58/023, grant to KS)
References
- 1.Mulero-Navarro S, Fernandez-Salguero PM. New Trends in Aryl Hydrocarbon Receptor Biology. Front Cell Dev Biol. 2016;4:45–45. doi: 10.3389/fcell.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, Cooke MP. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–8. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi KD, Vodyanik MA, Togarrati PP, Suknuntha K, Kumar A, Samarjeet F, Probasco MD, Tian S, Stewart R, Thomson JA, Slukvin II. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2(3):553–67. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108(6):2095–105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119(9):2818–29. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, Kaufman DS. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113(24):6094–101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, Sadelain M. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31(10):928–33. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vodyanik MA, Slukvin II. Hematoendothelial differentiation of human embryonic stem cells. Curr Protoc Cell Biol. 2007. Chapter 23:Unit 23.6. [DOI] [PubMed]
- 9.Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, Slukvin I. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27(3):559–67. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 11.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105(2):617–26. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 12.Togarrati PP, Suknuntha K. Generation of mature hematopoietic cells from human pluripotent stem cells. Int J Hematol. 2012;95(6):617–23. doi: 10.1007/s12185-012-1094-x. [DOI] [PubMed] [Google Scholar]
- 13.Faubert A, Chagraoui J, Mayotte N, Frechette M, Iscove NN, Humphries RK, Sauvageau G. Complementary and independent function for Hoxb4 and Bmi1 in HSC activity. Cold Spring Harb Symp Quant Biol. 2008;73:555–64. doi: 10.1101/sqb.2008.73.030. [DOI] [PubMed] [Google Scholar]
- 14.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109(1):39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- 15.Gori JL, Chandrasekaran D, Kowalski JP, Adair JE, Beard BC, D'Souza SL, Kiem HP. Efficient generation, purification, and expansion of CD34(+) hematopoietic progenitor cells from nonhuman primate-induced pluripotent stem cells. Blood. 2012;120(13):e35–44. doi: 10.1182/blood-2012-05-433797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thordardottir S, Hangalapura BN, Hutten T, Cossu M, Spanholtz J, Schaap N, Radstake TR, van der Voort R, Dolstra H. The aryl hydrocarbon receptor antagonist StemRegenin 1 promotes human plasmacytoid and myeloid dendritic cell development from CD34+ hematopoietic progenitor cells. Stem Cells Dev. 2014;23(9):955–67. doi: 10.1089/scd.2013.0521. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JE, Jr, Brunstein CG, Boitano AE, DeFor TE, McKenna D, Sumstad D, Blazar BR, Tolar J, Le C, Jones J, Cooke MP, Bleul CC. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell. 2016;18(1):144–55. doi: 10.1016/j.stem.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]