Abstract
Background and Aims
Biliary strictures after orthotopic liver transplantation (OLT) are typically managed by sequential ERCP procedures, with incremental dilation of the stricture and stent exchange (IDSE) and placement of new stents. This approach resolves >80% of strictures after 12 months, but requires costly, lengthy ERCPs with significant patient radiation exposure. Increasing awareness of the harmful effects of radiation, escalating health care costs, and decreasing reimbursement for procedures mandate maximal efficiency in performing ERCP. We compared the traditional IDSE protocol with a sequential stent addition (SSA) protocol, in which additional stents are placed across the stricture during sequential ERCPs, without stent removal/exchange or stricture dilation.
Methods
Patients undergoing ERCP for OLT-related anastomotic strictures from 2010–2016 were identified from a prospectively maintained endoscopy database. Procedure duration, fluoroscopy time, stricture resolution rates, adverse events, materials fees, and facility fees were analyzed for IDSE and SSA procedures.
Results
Seventy-seven patients underwent 277 IDSE and 132 SSA procedures. Mean fluoroscopy time was 64.5% shorter (p<0.0001) and mean procedure duration was 41.5% lower (p<0.0001) with SSA compared with IDSE. SSA procedures required fewer accessory devices, resulting in significantly lower material (63.8%, p<0.0001) and facility costs (42.8%, p<0.0001) compared with IDSE. Stricture resolution was >95% and low adverse event rates did not significantly differ.
Conclusions
Sequential stent addition results in shorter, cost-effective procedures requiring fewer accessory devices and exposing patients to less radiation. Stricture resolution rates are equivalent to incremental dilation and stent exchange and adverse events do not differ significantly, even in this immunocompromised population.
Keywords: ERCP, Biliary Stricture, Biliary Stent, Fluoroscopy, Radiation
Introduction
Post-operative bile duct strictures are seen after orthotopic liver transplant (OLT) and less commonly after cholecystectomy. Despite advances in surgical techniques, anastomotic biliary strictures develop in up to 12% of patients who undergo deceased donor liver transplants,1, 2 and represent the most common biliary adverse event seen after OLT.3 Advances in therapeutic endoscopy have led to endoscopic management replacing surgical repair as the standard of care.4, 5
Management of anastomotic biliary strictures after OLT typically comprises sequential endoscopic retrograde cholangiopancreatography (ERCP) procedures over a period of several months to a year. At the index ERCP, a sphincterotomy is performed, the stenosis is dilated and a plastic stent is placed across the stenosis.5 During subsequent ERCPs the previously placed stent(s) are removed, the stenosis is incrementally dilated, and an increasing number of plastic biliary stents are inserted. The plastic stents maintain dilation over the longer term until remodeling and resolution of the stricture occurs.6 This process of incremental dilation and stent exchange (IDSE) typically results in long-term stricture resolution in >85% of patients.7–9 Although reported success rates of this standard endotherapy approach are high, increasing awareness of the harmful effects of medical radiation exposure, along with escalating healthcare costs and decreasing procedural reimbursement, all advocate for the need to develop radiation-sparing endoscopic approaches that are more time and resource efficient.
Fluoroscopy used during ERCP exposes patients and endoscopy room personnel to ionizing radiation, with potentially harmful health effects. Over the last 3 decades, there has been a 6-fold increase in per capita medical radiation exposure in the United States.12 The long-term risk of medical radiation exposure is problematic, and it is estimated that up to 2% of all cancer may be attributable to radiation from CT scans alone.13 The amount of patient radiation exposure from a therapeutic ERCP is significant, and has been estimated at some centers to confer a lifetime risk of cancer of 1 in 1700, greater than the radiation exposure and cancer risk associated with a CT scan.14 The multiple ERCPs required for IDSE in the management of biliary strictures therefore result in significant cumulative patient radiation exposure over the year of endotherapy. Approaches to minimize patient radiation exposure over the course of these sequential ERCPs would result in an overall lower risk of adverse events in this immunosuppressed patient population, which is already predisposed to malignancy.15
An approach to decreasing patient radiation exposure, while simultaneously shortening the duration of ERCPs for biliary stricture management is to simply add a new biliary stent at each sequential ERCP, rather than to remove and replace all stents. Currently, standard practice is to remove all plastic stents and replace them with new plastic stents during each ERCP.5, 16 The rationale for removal and replacement of all previously placed stents at each ERCP is not evidence-based, but is driven primarily by the theoretical risk of stent occlusion and cholangitis in this high risk immunocompromised population.
To minimize patient and staff radiation exposure, maximize procedural efficiency, and minimize utilization of unnecessary accessory devices during ERCP, our institution discontinued its protocol of incremental dilation and stent exchange and in 2013 adopted a protocol of sequential stent addition instead. Under this new protocol, the previously placed stent(s) are left in place across the anastomotic stricture. During each sequential ERCP, performed over the course of approximately a year, an additional stent is inserted across the stricture. In this retrospective, single-center study at our tertiary care academic medical center, we compared the traditional incremental dilation and stent exchange with our sequential stent addition protocol and evaluated the impact of the new protocol on patient radiation exposure, procedural time, device related costs and facility fees.
Methods
Study Design
In this retrospective, single-center study we used our prospectively maintained endoscopy database to extract data on all patients who underwent ERCP for OLT-related biliary adverse events at our tertiary care academic medical center between 2010 and 2016. All patients who underwent OLT complicated by development of an anastomotic stricture were included in the study and none of the patients had undergone prior endotherapy for anastomotic stricture management.
Patients were grouped per their management approach: (1) incremental dilation and stent exchange (IDSE) or (2) sequential stent addition (SSA). A small number of patients were included in both study groups due to active treatment of these patients at the time of the change in institutional protocol from IDSE to SSA. For these patients, individual procedures were classified by the technique used. This study was conducted under a protocol approved by the Stanford Institutional Review Board (Protocol no. 36764).
Data Collection
Demographic data including age and gender were collected. Patients were assessed for stricture resolution at the end of the treatment period. They were subsequently followed clinically along with laboratory studies and imaging if indicated, to evaluate for stricture recurrence. Adverse events which occurred over the course of endoscopic therapy were documented and classified by type (cholangitis, stent dysfunction, stent migration, pancreatitis, bleeding, perforation). Cholangitis was defined as evidence of an infection originating within the biliary tract, typically manifesting with fever and other signs of local or systemic infection (leukocytosis, tachycardia, hypotension) and associated evidence of biliary obstruction (elevated liver function tests or radiographic evidence of biliary obstruction). Unplanned stent exchanges (defined as any stent exchange that occurred on a non-elective basis, or which for medical reasons was performed earlier than the date of planned follow up elective procedure) were also recorded. Unplanned stent exchanges were typically performed for presentation with suspected biliary obstruction or stent occlusion.
The ERCP procedure duration was calculated from time of scope insertion to time of scope removal. Accessory devices used in each procedure were recorded and cumulative cost of devices was calculated for each independent procedure based on listed cost for individual devices. Fluoroscopy time was also recorded for each procedure. Fellow participation in each procedure was also documented.
Endoscopy room utilization time was calculated from the time of patient entry into the endoscopy room to time of exit from the room (“wheels in to wheels out”). In addition to the endoscopic procedure itself, this room utilization time incorporates pre-procedural elements such as immediate pre-anesthesia assessment, initiation of anesthesia, patient positioning on fluoroscopy table, final verifications, termination of anesthesia and immediate postprocedure care. Duration of time in the endoscopy room for any given procedure was then used to calculate facility fees based on our institution’s standard billing practice. Billing of facility fees is based on a flat rate for the first 30 minutes of the procedure, with incremental increase in cost for each additional 5 minutes of procedure time.
Endoscopy protocol
ERCPs were performed using standard techniques with the patient under monitored anesthesia care or general anesthesia as determined by the anesthesiologist. Prophylactic antibiotics were administered to all patients. When an anastomotic stricture was detected at the index procedure, sphincterotomy was typically performed and a plastic stent was advanced across the stricture, with selection of stent diameter based on the diameter of the bile duct at the level of the anastomosis. Repeat ERCPs were scheduled every 2.5 to 3 months on an elective basis for stent addition or exchange in both groups of patients.
Incremental Dilation and Stent Exchange
For IDSE procedures, subsequent ERCPs involved removal of previously placed plastic biliary stents, followed by selective biliary cannulation with cholangiogram to assess the anastomosis. Balloon dilation of the stenosis was typically performed and extraction of stent related sludge/stone debris was undertaken if present. The number of stents was incrementally increased with each ERCP, until the final ERCP when all stents were removed.
Sequential Stent Addition
For SSA procedures, subsequent ERCPs involved selective biliary cannulation alongside previously placed biliary stents, and advancement of a guidewire across the stricture. A cholangiogram was performed to assess the degree of residual stenosis and room for additional stent placement in the common bile and hepatic ducts (Figure 1). An additional stent was then advanced across the anastomosis alongside the stent(s) placed during previous procedure(s). The previously placed biliary stent(s) were not removed during these procedures and balloon dilation typically was not performed, as the leading end of the stent delivery system provided adequate dilation for advancement of a single stent across the anastomosis.
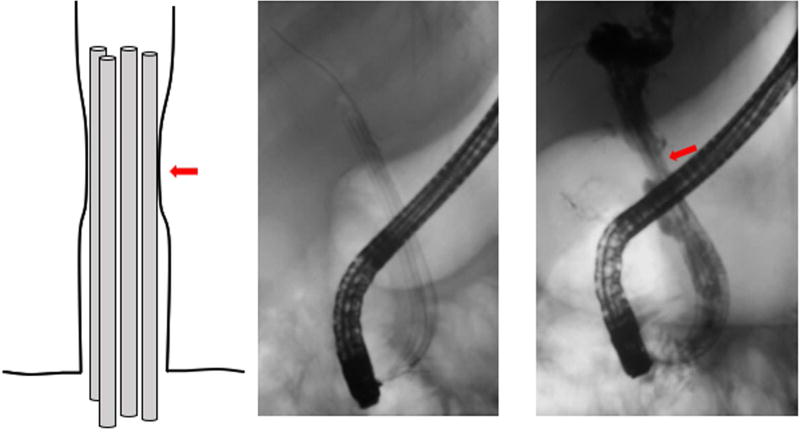
Figure 1.
Despite multiple stents extending across the anastomosis, contrast injection reveals a “waist” (red arrows) at the level of the stricture, enabling assessment of space available for placement of additional stent(s). Stents are added at sequential procedures until this waist largely resolves.
Statistical Analysis
All analyses were conducted using SAS Enterprise Guide version 7.11 HF3 (SAS Institute Inc., Cary, NC). Continuous data were analyzed for normality using the Kolmogorov-Smirnov test. Continuous data where a single observation was measured per patient was analyzed using Student’s t-test. Continuous data where repeated observations were measured per patient were analyzed using generalized estimating equations and robust (empirical) standard errors to account for patient-level clustering effect. Binary outcomes with five or greater events per cohort were analyzed with the χ2 test, or with generalized estimating equations with a link to logit function and robust standard errors if repeated observations were measured per patient. Binary outcomes with fewer than five events per cohort were analyzed per the Fisher’s exact test for sparsely distributed data. All reported p-values are 2-sided, and all comparisons attained statistical significance at p < 0.05.
Results
A total of 410 ERCPs were performed by 5 therapeutic endoscopists on 77 patients for endotherapy of anastomotic strictures after OLT with choledocho-choledochostomy. ERCPs performed included 277 IDSE procedures performed on 45 patients and 132 SSA procedures performed on 32 patients. Median duration of endotherapy (time from index to final ERCP) was 13 months for IDSE patients and 11 months in the SSA patients. The mean age of patients was 62.1 years (SD 6.0 years) in the IDSE group and 56.2 years (SD 9.3 years) in the SSA group (p=0.002)(Table 1). The IDSE group comprised 31.1% female patients and the SSA group comprised 31.3% female patients (p=0.89) (Table 1). Fellows participated in 68.4% of IDSE procedures and in 94.7% of SSA procedures.
Table 1.
Patient characteristics
| # Patients |
# Procedures |
Age in years (SD) |
% Female Gender |
Maximal stent bundle size in French (SD) |
% Patients with Bilirubin > 3 prior to Index ERCP |
% Patients administered rectal Indomethacin |
% Patients with PD stent placement |
|
|---|---|---|---|---|---|---|---|---|
| Sequential Stent Addition | 32 | 132 | 56.2 (9.3) | 31.3 | 33.3 (8.4) | 32.0% | 72.0% | 4.0% |
| Incremental Dilation & Stent Exchange | 45 | 277 | 62.1 (6.0) | 31.1 | 33.8 (8.3) | 28.9% | 15.4% | 11.5% |
| P value | 0.002 | 0.89 | 0.99 | 0.78 | <0.0001 | 0.42 |
Procedure Duration
The mean procedure duration for all IDSE procedures from the index stent insertion to the final removal of all stents was 29.32 (SD 5.73) minutes. In contrast, the mean procedure duration for all SSA procedures was only 17.15 (SD 5.54) minutes (p<0.0001), reflecting a 41.5% shorter procedure time (Table 2). The procedure duration for the index and final ERCPs can be expected to be similar in both IDSE and SSA groups, as both follow the same procedure protocol of placement of a single stent (index ERCP) or removal of all stents (final ERCP). We therefore evaluated procedure duration for the intervening procedures. The mean procedure duration for the intervening procedures (after exclusion of the first and final ERCPs) was 27.55 (SD 6.17) minutes for IDSE. It was significantly lower for SSA procedures at 10.35 (SD 5.98) minutes (p<0.0001) reflecting a 62.4% shorter procedure time (Table 3).
Table 2.
Procedure parameters (all ERCPs)
| All ERCPs | Procedure Duration in minutes (SE) |
Fluoroscopy Time in minutes (SE) |
Materials Cost per procedure in $ (SE) |
Facility Cost per procedure in $ (SE) |
|---|---|---|---|---|
| Sequential Stent Addition | 17.15 (5.54) | 1.22 (0.42) | 274.3 (21.6) | 11,118.9 (3,894.9) |
| Incremental Dilation & Stent Exchange | 29.32 (5.73) | 3.43 (0.44) | 759.4 (83.8) | 19,450.3 (4000.4) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Table 3.
Procedure parameters (excluding index and final ERCPs)
| Excluding index and final ERCPs |
Procedure Duration in minutes (SE) |
Fluoroscopy Time in minutes (SE) |
Materials Cost per procedure in $ (SE) |
Facility Cost per procedure in $ (SE) |
|---|---|---|---|---|
| Sequential Stent Addition | 10.35 (5.98) | 0.68 (0.51) | 407.7 (93.5) | 8,436.0 (4849.3) |
| Incremental Dilation & Stent Exchange | 27.55 (6.17) | 3.41 (0.53) | 757.8 (96.8) | 20,709.6 (4,952.2) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Fluoroscopy Time (FT)
Mean FT per IDSE procedure was 3.43 (SD 0.44) minutes. The mean FT was significantly lower for SSA procedures at 1.22 (SD 0.42) minutes (p<0.0001, Table 2) for an overall 64.5% decrease in FT with implementation of the SSA protocol. Evaluation of FT for intervening procedures (after exclusion of index and final ERCPs) indicated an even more pronounced 80.1% decrease in FT for SSA procedures compared with IDSE procedures (3.41 ± 0.53 vs 0.68 ± 0.51, p<0.0001, Table 3). FT for intervening SSA procedures was therefore 5-fold lower than for IDSE procedures.
Cost of Accessory Devices
Mean cost of devices and instruments for each IDSE procedure was $759.40 (SD $83.80) and this cost was 63.8% lower in SSA procedures, at $274.30 (SD $21.60, p<0.0001, Table 2). For the intervening procedures (after exclusion of index and final ERCPs), the mean device cost for IDSE procedures was $757.80 (SD $96.80), and the cost was 46.2% lower in SSA procedures, at $407.70 (SD $93.50, p<0.0001, Table 3).
Fluoroscopy Room Utilization
Mean overall “in room” (wheels in to wheels out) time was 46.3 minutes (SD 14.3) for IDSE procedures and significantly shorter at 32.4 minutes (SD 10.7) for SSA procedures (p<0.0001). This resulted in a 42.8% lower mean facility fee for SSA procedures compared with IDSE procedures (p<0.0001, Table 2). For the intervening procedures (after exclusion of index and final ERCPs) the difference was more pronounced, with a 59.3% lower facility fee for SSA procedures compared with IDSE procedures (p<0.0001, Table 3).
Outcomes
Initial stricture resolution was achieved in 100% of patients who completed endotherapy in both IDSE and SSA groups. The mean final cumulative diameter (French) of stents was 33.3 in the SSA group and 33.8 in the IDSE group (p=0.99, Table 1). The minimum period of follow up for all patients was 6 months (range 7 months to 88 months). Over the follow-up period, anastomotic strictures recurred in 3 patients (two in IDSE group, one in SSA group). SSA patients therefore had a 96.9% rate of durable stricture resolution, whereas IDSE patients had a 95.6% rate of durable stricture resolution over the duration of follow-up (p=0.8060).
Adverse Events
Cholangitis developed after 4 of 271 IDSE procedures (1.4%) and no patients in the SSA group developed cholangitis (p=0.31 Table 4). Unplanned stent exchanges occurred at a rate of 3.6% for IDSE procedures and 0.8% for SSA procedures (p=0.11). Stent migration was observed in 3.6% of IDSE procedures and in 5.3% of SSA procedures (p=0.18, Table 4). ERCPs in the IDSE cohort were complicated by bleeding at a rate of 1.1% (3 procedures) and none of the SSA procedures resulted in bleeding (p=0.55, Table 4). Pancreatitis did not develop in any patient in either study group. Perforation was not observed in procedures for either study group.
Table 4.
Adverse Events
| Cholangitis | Bleeding | Perforation | Pancreatitis | Stent migration | Unplanned stent exchange |
Any complication | |
|---|---|---|---|---|---|---|---|
| Sequential Stent Addition | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (5.3%) | 1 (0.8%) | 8 (6.1%) |
| Incremental Dilation & Stent Exchange | 4 (1.4%) | 3 (1.1%) | 0 (0%) | 0 (0%) | 10 (3.6%) | 10 (3.6%) | 22 (7.9%) |
| P Value | 0 31 | 0.55 | -- | -- | 0.18 | 0.11 | 0.17 |
Discussion
The evolution in the management of post-operative biliary strictures from surgery to endotherapy is a testament to advancing technology and the expanding therapeutic capability of endoscopy. Although endoscopy is less invasive than surgery, with significantly lower mortality and morbidity rates, a disadvantage is the need for repeated endoscopic procedures over a relatively protracted treatment period. Additionally, the multiple ERCPs required to achieve stricture resolution result in significant cumulative radiation exposure for patients. Ionizing radiation is associated with genomic instability and an increased risk of developing cancer,10, 11 with mounting evidence that there is a linear increase in cancer mortality with increased exposure to ionizing radiation.17 As patient outcomes after endotherapy are already very favorable with stricture resolution rates of over 80%,18 further refinement of endoscopic therapy should ideally minimize the undesirable consequences of ERCP including radiation exposure, while improving procedural efficiency.
Several endoscopic approaches have been evaluated for the management of bile duct strictures. Balloon dilation of strictures without stent placement has been undertaken, but is durably successful in only approximately 40% of patients with anastomotic strictures.19 Incremental dilation with stenting has become the de facto standard of care with higher success rates associated with stent placement for ≥12 months.7 The reasoning behind this approach has been hypothetical minimization of the risk of stent occlusion-related cholangitis in this high risk immunocompromised population. However, no data exist to support this reasoning. Covered metallic stents have been used in some studies for management of post-OLT anastomotic biliary stricture,20 but are associated with disadvantages including high stent migration rates,21, 22 increased pancreatitis rates,23 bile leaks,21 and a risk of secondary stricture formation,24 all of which have prevented this approach from becoming the standard of care.
Minimizing patient radiation exposure during ERCP has been a major research and quality assurance focus of our interventional endoscopy group.25–29 We have traditionally managed post-transplant anastomotic strictures with IDSE performed for a period of approximately 12 months. Then in 2013, in an effort to minimize patient radiation exposure, we conceptualized and implemented a SSA protocol for anastomotic biliary strictures after OLT. Only a single patent stent is required to allow biliary drainage and moreover, in the presence of 2 or more stents, several additional drainage channels are created between stents (Figure 2). We therefore hypothesized that this approach would allow for adequate biliary drainage, shorten the procedure time and minimize radiation exposure to patients and endoscopy room staff.
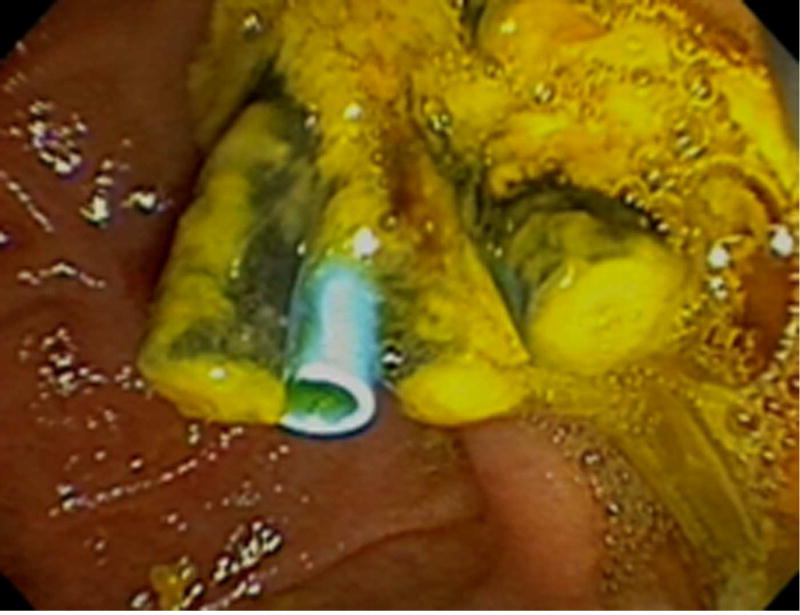
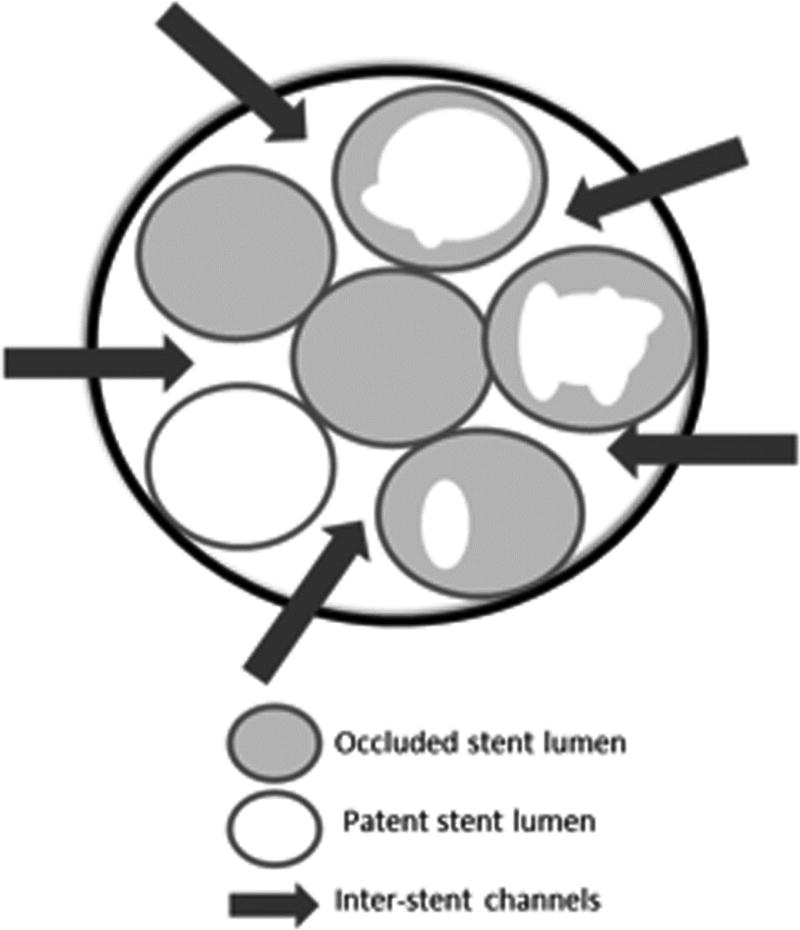
Figure 2.
A. Endoscopic image of a newly placed plastic biliary stent alongside previously placed occluded and partially occluded biliary stents at the ampulla of Vater. B. Schematic demonstrating multiple inter-stent channels between patent, partially occluded and occluded biliary stents.
Our study indicated no difference in rates of stricture resolution, stricture recurrence or adverse events between the two endoscopic approaches and no difference in adverse event rates. These findings suggest that contrary to widespread assumptions, the IDSE elements of removal of all previously placed (potentially occluded) stents and replacement with new stents during each procedure, do not decrease the risk of cholangitis. Moreover, extraction of sludge and stone debris during IDSE procedures does not decrease the risk of cholangitis. We found no increase in cholangitis, unplanned stent exchanges, or stent migration with the SSA approach. Our data clearly indicate that addition of a single new stent during each procedure suffices to maintain safe biliary drainage even in this high-risk group of immunocompromised patients.
Despite use of pre-procedure prophylactic antibiotics in both groups, we noted 4 patients with cholangitis with the older IDSE approach and none with the new SSA approach. Although this did not reach statistical significance, this trend may be worth reflecting upon, given the grave consequences of cholangitis in this immunocompromised population. The biliary tree is inevitably colonized with bacteria after index ERCP, and the duration and degree of instrumentation during subsequent ERCPs may play a role in the likelihood of developing cholangitis. The IDSE approach, with its multiple sequential steps may therefore increase this risk, compared with the solitary step of single stent placement with the new SSA approach. Additionally, contrast injection typically performed with an occluding balloon catheter in IDSE patients also raises intra-biliary pressures thereby potentially increasing the risk of cholangitis. Conversely, the SSA approach may not result in a significant increase in intra-biliary pressures while obtaining a cholangiogram, due to free and rapid outflow of injected contrast from the bile duct through the indwelling previously placed stents and adjacent inter-stent channels.
Our primary motivation in transitioning to the new SSA protocol was to decrease patient radiation exposure in these patients who undergo several ERCPs to accomplish resolution of biliary strictures. With adoption of the SSA protocol, we noted a significant 64.5% decrease in mean fluoroscopy time. During the entire course of endotherapy spread over a year, transitioning from IDSE to SSA would decrease extrapolated cumulative fluoroscopy time from 17.1 to 4.5 minutes. Several in vitro and in vivo experiments have demonstrated that exposure to ionizing radiation increases the risk of developing some types of cancer.10, 30 Whereas concentrated high dose rate radiation exposure was previously thought to confer a higher cancer risk than low dose rate exposure,31 a recent retrospective cohort study of nuclear workers chronically exposed to low dose radiation provided evidence for a linear increase in cancer mortality with increased exposure to ionizing radiation, regardless of the radiation dose rate.17 This estimated 64.5% decrease in fluoroscopy time and the associated radiation exposure is substantial and may decrease malignancy risk in patients undergoing serial ERCPs for biliary stricture management.
In addition to decreased radiation exposure, we noted several benefits with the SSA approach, including a shorter procedure duration, requirement for fewer endoscopic accessory devices, and consequently lower materials and facility fees. The mean procedure duration (time from scope insertion to scope removal) decreased by 41.5% with transition to the SSA protocol. This increased procedural efficiency should allow endoscopy units to schedule additional procedures each day, resulting in improved endoscopy unit efficiency. The shorter SSA procedures may also be more amenable to monitored anesthesia care rather than to full general anesthesia with intubation, which may potentially further decrease patient ‘in room’ time as well as anesthesia-associated fees. Furthermore, the reduced procedure time requires shorter periods of sedation/anesthesia, which have been shown to be associated with fewer anesthesia-related adverse events and more favorable patient outcomes after endoscopic procedures.15
Procedure duration is directly linked to facility fees at many institutions, including ours. Time in the procedure room (“wheels in to wheels out”) was significantly lower in SSA procedures, resulting in a 42.8% decrease in facility fees for SSA procedures compared with IDSE procedures. Additionally, the simpler, shorter SSA procedure required fewer endoscopic accessory devices due to elimination of the need for snares to remove previously placed stents, stone extraction balloons and stricture dilation balloons as well as use of fewer stents and stent delivery systems. This use of fewer devices in SSA procedures resulted in a 63.8% decrease in the cost of materials relative to IDSE procedures. In this era of escalating healthcare costs and decreasing procedural reimbursement, the SSA approach offers several significant efficiencies including shorter procedure times, lower device costs and lower anesthesia and facility fees.
Limitations of the study include its retrospective nature and temporal grouping of stent addition and exchange procedures. Stent exchange procedures were performed from 2010 to 2013 and stent addition procedures were performed from 2013 to 2016. As a consequence of this temporal grouping, the duration of follow-up for the IDSE group is longer compared with the SSA group. With the emergence of data supporting use of rectal Indomethacin for post-ERCP pancreatitis prophylaxis, we began using this in 2012 and this shift in our practice is reflected in the significant increase in Indomethacin administered to SSA patients relative to IDSE patients. The median number of ERCPs per patient and overall duration of endotherapy was lower in the SSA relative to the IDSE approach, which reflects an evolution of our institutional approach to management of anastomotic biliary strictures over time. However, despite the shorter median duration of stenting with SSA compared with the IDSE approach, strictures in both groups were dilated to the same diameter and we noted no significant difference in stricture resolution rates. Trainee participation was not uniform between the IDSE and SSA groups. Trainees participated in approximately two-thirds of IDSE procedures and in almost all SSA procedures. Fellow involvement may be expected to prolong procedure duration and increase fluoroscopy use; nevertheless we noted a significant decrease in both procedure duration and FT for SSA procedures despite the higher proportion of these procedures performed with trainees. Minimizing patient radiation exposure has been a goal of our endoscopy unit, and it is possible that these background efforts over the years may have contributed to the reduction in fluoroscopy time with SSA implementation. In terms of adverse events, amylase levels are not routinely drawn on all patients after the procedure, and it is possible that mild pancreatitis may not have been detected if a patient did not report symptoms immediately after the procedure or upon 24-hour follow-up. Finally, our cost analysis is limited to procedure and facility fees directly incurred during endotherapy and does not take longer-term costs into account.
In conclusion, this study, performed in the highest risk immunocompromised patients with biliary strictures indicates that the SSA protocol shows similar outcomes and no increase in adverse events compared with the standard IDSE protocol, but is associated with significantly lower patient radiation exposure, decreased procedure time and decreased procedure costs. Further similar studies at other centers will be beneficial in validating our results. We have applied this stent addition protocol to other benign biliary strictures, including post-pancreatitis and post-cholecystectomy strictures, with similar success rates, indicating our data may be generalized to a multitude of routine stent exchange procedures. Our robust results make an argument for other institutions to consider adopting the SSA approach for the management of bile duct strictures.
Acknowledgments
Financial Support:
This work was supported by a NIH T32 Training Grant (DK007056) supporting MTB and RJH
Acronyms
- OLT
Orthotopic Liver Transplant
- ERCP
Endoscopic Retrograde Cholangio-pancreatography
- IDSE
Incremental Dilation and Stent Exchange
- SSA
Sequential Stent Addition
- SD
Standard Deviation
- FT
Fluoroscopy Time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None of the authors have any conflicts of interest pertaining to the study to disclose. All research was conducted in accordance with appropriate ethical guidelines.
Oral presentation at DDW.
Author Contributions:
NT and SB were involved in conception and design of the study; MTB, RJH, NT, AC and SB were involved in collection, analysis and interpretation of the data; MTB, RJH, MG and SB were involved in drafting and critical revision of the article for important intellectual content; SB granted final approval of the article.
References
- 1.Ayoub WS, Esquivel CO, Martin P. Biliary complications following liver transplantation. Dig Dis Sci. 2010;55:1540–6. doi: 10.1007/s10620-010-1217-2. [DOI] [PubMed] [Google Scholar]
- 2.Krok KL, Cardenas A, Thuluvath PJ. Endoscopic management of biliary complications after liver transplantation. Clin Liver Dis. 2010;14:359–71. doi: 10.1016/j.cld.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Seehofer D, Eurich D, Veltzke-Schlieker W, et al. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253–65. doi: 10.1111/ajt.12034. [DOI] [PubMed] [Google Scholar]
- 4.Gomes RM, Doctor NH. Predictors of outcome after reconstructive hepatico-jejunostomy for post cholecystectomy bile duct injuries. Trop Gastroenterol. 2015;36:229–35. doi: 10.7869/tg.296. [DOI] [PubMed] [Google Scholar]
- 5.Costamagna G, Pandolfi M, Mutignani M, et al. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointestinal Endoscopy. 2001;54:162–168. doi: 10.1067/mge.2001.116876. [DOI] [PubMed] [Google Scholar]
- 6.Perri V, Familiari P, Tringali A, et al. Plastic biliary stents for benign biliary diseases. Gastrointest Endosc Clin N Am. 2011;21:405–33. doi: 10.1016/j.giec.2011.04.012. viii. [DOI] [PubMed] [Google Scholar]
- 7.Kao D, Zepeda-Gomez S, Tandon P, et al. Managing the post-liver transplantation anastomotic biliary stricture: multiple plastic versus metal stents: a systematic review. Gastrointest Endosc. 2013;77:679–91. doi: 10.1016/j.gie.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Nacif LS, Bernardo WM, Bernardo L, et al. Endoscopic treatment of post-liver transplantation anastomotic biliary stricture: systematic review and meta-analysis. Arq Gastroenterol. 2014;51:240–9. doi: 10.1590/s0004-28032014000300014. [DOI] [PubMed] [Google Scholar]
- 9.Peng C, Ma C, Xu G, et al. The Efficacy and Safety of Endoscopic Balloon Dilation Combined with Stenting in Patients with Biliary Anastomotic Strictures After Orthotopic Liver Transplantation. Cell Biochem Biophys. 2015;72:385–97. doi: 10.1007/s12013-014-0473-8. [DOI] [PubMed] [Google Scholar]
- 10.Bouffler S, Silver A, Cox R. Mechanistic and genetic studies of radiation tumorigenesis in the mouse--implications for low dose risk estimation. J Radiol Prot. 2002;22:A11–6. doi: 10.1088/0952-4746/22/3a/302. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe D, Bowden GT. Ionizing radiation as an initiator: effects of proliferation and promotion time on tumor incidence in mice. Cancer Res. 1987;47:6692–6. [PubMed] [Google Scholar]
- 12.Mettler FA, Jr, Bhargavan M, Faulkner K, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources-1950–2007. Radiology. 2009;253:520–31. doi: 10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 13.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 14.Larkin CJ, Workman A, Wright RE, et al. Radiation doses to patients during ERCP. Gastrointest Endosc. 2001;53:161–4. doi: 10.1067/mge.2001.111389. [DOI] [PubMed] [Google Scholar]
- 15.Agostoni M, Fanti L, Gemma M, et al. Adverse events during monitored anesthesia care for GI endoscopy: an 8-year experience. Gastrointest Endosc. 2011;74:266–75. doi: 10.1016/j.gie.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Morelli J, Mulcahy HE, Willner IR, et al. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc. 2003;58:374–9. doi: 10.1067/s0016-5107(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 17.Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS) BMJ. 2015;351:h5359. doi: 10.1136/bmj.h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DW, Jo HH, Abdullah J, et al. Endoscopic Management of Anastomotic Strictures after Liver Transplantation. Clin Endosc. 2016;49:457–461. doi: 10.5946/ce.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoepf T, Maldonado-Lopez EJ, Hilgard P, et al. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl. 2006;12:88–94. doi: 10.1002/lt.20548. [DOI] [PubMed] [Google Scholar]
- 20.Cote GA, Slivka A, Tarnasky P, et al. Effect of Covered Metallic Stents Compared With Plastic Stents on Benign Biliary Stricture Resolution: A Randomized Clinical Trial. JAMA. 2016;315:1250–7. doi: 10.1001/jama.2016.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahaleh M, Behm B, Clarke BW, et al. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video) Gastrointest Endosc. 2008;67:446–54. doi: 10.1016/j.gie.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 22.Tal AO, Finkelmeier F, Filmann N, et al. Multiple Plastic Stents Versus Covered Metal Stent for Treatment of Anastomotic Biliary Strictures after Liver Transplantation: a Prospective, Randomized, Multicenter Trial. Gastrointest Endosc. 2017 doi: 10.1016/j.gie.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Martins FP, Kahaleh M, Ferrari AP. Management of liver transplantation biliary stricture: Results from a tertiary hospital. World J Gastrointest Endosc. 2015;7:747–57. doi: 10.4253/wjge.v7.i7.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaput U, Vienne A, Audureau E, et al. Temporary placement of fully covered self-expandable metal stents for the treatment of benign biliary strictures. United European Gastroenterol J. 2016;4:403–12. doi: 10.1177/2050640615606550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao C, Thosani N, Kothari S, et al. Radiation exposure to patients during ERCP is significantly higher with low-volume endoscopists. Gastrointest Endosc. 2015;81:391–8. doi: 10.1016/j.gie.2014.08.001. e1. [DOI] [PubMed] [Google Scholar]
- 26.Sethi S, Thosani N, Banerjee S. Radiation-Free ERCP in Pregnancy: A "Sound" Approach to Leaving No Stone Unturned. Dig Dis Sci. 2015;60:2604–7. doi: 10.1007/s10620-014-3502-y. [DOI] [PubMed] [Google Scholar]
- 27.Sethi S, Friedland S, Banerjee S. U.S. Survey Assessing Current ERCP-Related Radiation Protection Practices. Gastrointest Endosc. 2015;81:AB352. [Google Scholar]
- 28.Thosani N, Chen AM, Friedland S, Banerjee S. Prospective Evaluation of Predictors of Increased Patient Radiation During ERCP: the Stanford ERCP Radiation Safety (Sers) Study. Gastrointestinal Endoscopy. 2014;79(5):AB340–AB341. [Google Scholar]
- 29.Thosani N, Wang J, Damavandi S, Chen AM, Friedland S, Banerjee S. Impact of Fluoroscopy Frame Rate, Image Magnification, Collimation and Height of the Image Intensifier on Radiation Exposure to Patients During ERCP. Gastrointest Endoscopy. 2014;79:AB341. [Google Scholar]
- 30.Cardis E, Vrijheid M, Blettner M, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ. 2005;331:77. doi: 10.1136/bmj.38499.599861.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardis E, Gilbert ES, Carpenter L, et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res. 1995;142:117–32. [PubMed] [Google Scholar]