Summary
The clearance of mitochondria by autophagy, mitophagy, is important for cell and organism health [1], and known to be regulated by ubiquitin. During Drosophila intestine development, cells undergo a dramatic reduction in cell size and clearance of mitochondria that depends on autophagy, the E1 ubiquitin-activating enzyme Uba1 and ubiquitin [2]. Here we screen a collection of putative ubiquitin binding domain encoding genes for cell size reduction and autophagy phenotypes. We identify the ESCRT components TSG101 and Vps36, as well as the novel gene Vps13D. Vps13D is an essential gene that is necessary for autophagy, mitochondrial size, and mitochondrial clearance in Drosophila. Interestingly, a similar mitochondrial phenotype is observed in VPS13D mutant human cells. The UBA domain of Vps13D binds K63 ubiquitin chains, and mutants lacking the UBA domain have defects in mitochondrial size and clearance, and exhibit semi-lethality, highlighting the importance of Vps13D ubiquitin-binding in both mitochondrial health and development. VPS13D mutant cells possess phosphorylated DRP1 and MFF, as well as DRP1 association with mitochondria, suggesting that VPS13D functions downstream of these known mitochondrial fission factors. In addition, the large Vps13D mitochondrial and cell size phenotypes are suppressed by decreased mitochondrial fusion gene function. Thus, these results provide a previously unknown link between ubiquitin, mitochondrial size regulation and autophagy.
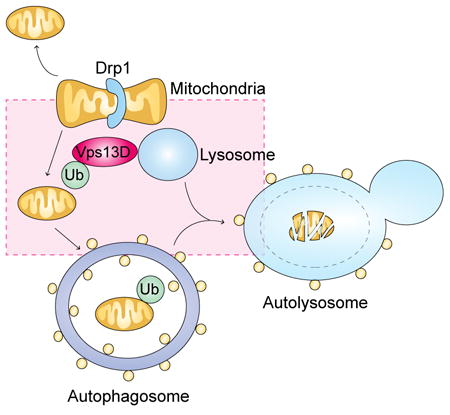
Graphical abstract
Results and Discussion
The function of Uba1 and ubiquitin in mitochondrial clearance and autophagy [2] prompted us to screen for genes that encode putative ubiquitin binding domain proteins that are required for these processes in the Drosophila intestine. We obtained UAS-regulated double-stranded inverse repeat RNAi lines against genes that encode putative ubiquitin-binding proteins (Table S1) and induced knockdown cell clones in midguts expressing the autophagy reporter mCherry-Atg8a in all cells. Examination of midguts 2 h after puparium formation allowed us to assay both for defects in cell size reduction and in Atg8a puncta formation. Among the 133 putative ubiquitin-binding protein encoding genes that we evaluated, known ubiquitin binding mitophagy receptors, such as p62 (Ref(2)P in flies) and Optineurin (Kenny, or key, in flies), did not exhibit a phenotype. For example, ref(2)p mutant midgut cells exhibited similar amounts of mitochondria as control midgut cells 2 h after puparium formation (Figure S1A-C), and ref(2)p knockdown (Figure S1D-D”) failed to block both programmed cell size reduction and mCherry-Atg8a puncta formation (Figure S1E and G). Significantly, we identified 3 genes, TSG101, Vps36 and CG32113, that exhibited a defect in cell size reduction and Atg8a puncta formation. TSG101 and Vps36 are components of the ESCRT pathway that were both required for programmed cell size reduction and exhibited a decrease in Atg8a puncta formation (Figure S2A-F). The ESCRT pathway has been previously shown to influence macroautophagy [3] and, thus, we decided to pursue the previously undescribed gene CG32113.
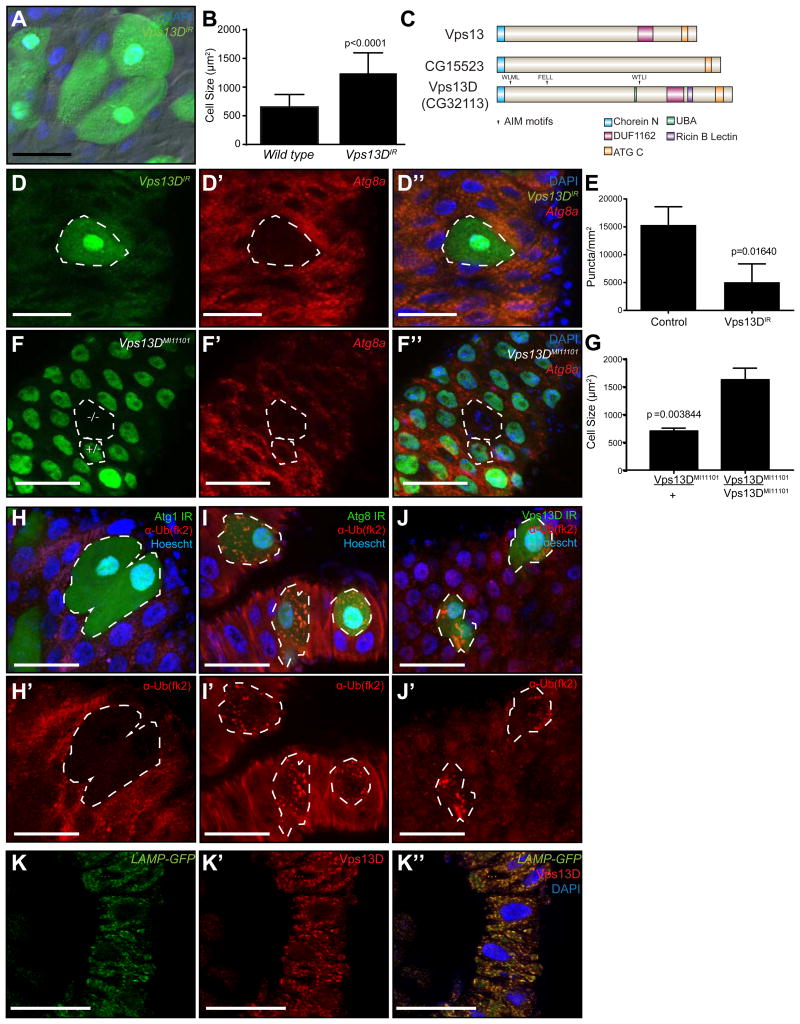
CG32113 (Vps13D) was found to be necessary for cell size reduction and Atg8a puncta formation in the midgut (Figure 1A, B, D and E). Significantly, a second unique Vps13D RNAi line that targets a distinct sequence, and a loss-of-function MiMIC insertion line (Vps13DMI11101) in which exon 13 of Vps13D is disrupted by the transgenic insertion of a Minos-based MiMIC construct [4], also influenced cell size reduction and Atg8a puncta formation (Figure 1F-G; Figure S2G-I). Knockdown of Vps13D in either the starved fat body (Figure S2J-K) or in intestines from rapamycin-fed larvae (Figure S2L-M), however, had no influence on mCherry-Atg8a puncta formation, indicating that Vps13D is not necessary for starvation-induced autophagy. Additionally, feeding larvae H2O2 induced Atg8a puncta formation that was not impaired by Vps13D knockdown (Figure S2N-O), further suggesting that Vps13D acts specifically in developmental autophagy in the midgut. Vps13D is a member of the Vps13 family of proteins, the members of which consist of Vps13 (similar to human Vps13A and Vps13C), CG15523 (similar to human Vps13B), and CG32113 in Drosophila (Figure 1C). Neither Vps13 nor CG15523 were required for either cell size reduction or mCherry-Atg8a puncta formation in the midgut of the intestine (Figure S2P-U). Because Vps13D is the only family member with a putative ubiquitin binding UBA domain, this suggests a possible role for the Vps13D UBA domain in autophagy.
Figure 1. Vps13D functions in programmed cell size reduction and Atg8 puncta formation in the Drosophila intestine.
(A) Control and Vps13D knockdown (green) cells in the Drosophila midgut stained with DAPI (blue).
(B) Quantitation of control wild type and Vps13D knockdown cell size from at least 76 cells in at least 9 intestines of either control or knockdown cell clones.
(C) Schematic of the Vps13 family. Drosophila Vps13D is the only Vps13 family member with a UBA domain. Atg8 interacting motifs analyzed in the manuscript are marked with arrows.
(D-D”) Clonal knockdown of Vpsl3D (green cells) impairs mCherry-Atg8a puncta formation (red).
(E) Quantitation of mCherry-Atg8a puncta in control and Vpsl3D knockdown cell clones from at least 28 clones in 4 intestines.
(F-F”) MiMIC insertion Vpsl3D mutant cell clones phenocopy Vpsl3D knockdown failure in cell size reduction and mCherry-Atg8a autophagy reporter puncta formation (red). The mutant clone (-/-; top cell) and an example of a heterozygous control cell (+/-; bottom cell) are outlined in white.
(G) Quantitation of Vpsl3D mutant and heterozygous control (green) cell size from clones from three intestines.
(H-J') (H-H'), Atg8a (I-I'), and Vpsl3D (J-J') knockdown (green) cells in the Drosophila midgut stained with DAPI (blue) and ubiquitin (fk2) antibody (red). Results are representative of at least three intestines per genotype.
(K-K”) Intestines expressing LAMP-GFP (green) were dissected 2h after puparium formation and stained with DAPI (blue) and Vpsl3D antibody (red). Results are representative of at least three intestines. Scale bars in all images represent 50um.
See also: Figures SI and S2, Table SI
Our data indicate that Vps13D is required for autophagy. To interrogate the possible relationship between the Vps13D ubiquitin binding protein, ubiquitin and autophagy, we investigated ubiquitin localization in Atg1, Atg8a, and Vps13D knockdown cells. Atg1 knockdown cells lacked ubiquitin puncta that existed in control neighboring cells (Figure 1H, H’), suggesting that this upstream kinase is required for a change in ubiquitination that is associated with autophagy in intestine cells. By contrast, intestine cells with reduced Atg8a function possessed ubiquitin puncta, but these puncta were enlarged compared to neighboring control cells (Figure 1I, I’). Importantly, Vps13D knockdown intestine cells also possessed enlarged ubiquitin puncta (Figure 1J, J’). These data suggest that Vps13D functions downstream of Atg1 to mediate a change in ubiquitin that is associated with both control of mitochondrial morphology and autophagy.
The function of Vps13D in cell size reduction and autophagy prompted us to consider the localization of this novel protein. Monoclonal antibodies were raised against Vps13D, and an antibody was identified that possessed cytoplasmic immune reactivity in tissues that is lost in Vps13D RNAi knockdown cells (Figure S3A-A”). Significantly, Vps13D co-localized with the lysosomal marker LAMP1-GFP, but did not exhibit a clear localization with either autophagosome or mitochondrial markers (Figure 1K-K”; Figure S3B-C”). Vps13D localization with a lysosomal-associated protein is interesting, as this provides a link between this protein and the ESCRT-associated genes that we identified in our screen that are known to influence late endosomes and lysosome dynamics [5].
Little is known about the function of Vps13D, but it was recently found to be an essential gene in human cell lines [6, 7]. Accordingly, constitutive expression of Vps13D RNAi by combining actin-Gal4 with UAS-Vps13DIR was 100% lethal at either larval or pupal stages, depending on the RNAi line used (data not shown). The loss-of-function MiMIC insertion line Vps13DMI11101 was also found to be 100% homozygous lethal (n = 144). In addition, Vps13DMI11101 combined with a deficiency for this region, Df(3L)BSC613, was also 100% lethal (n = 146). These data indicate that Vps13D is essential in the fly.
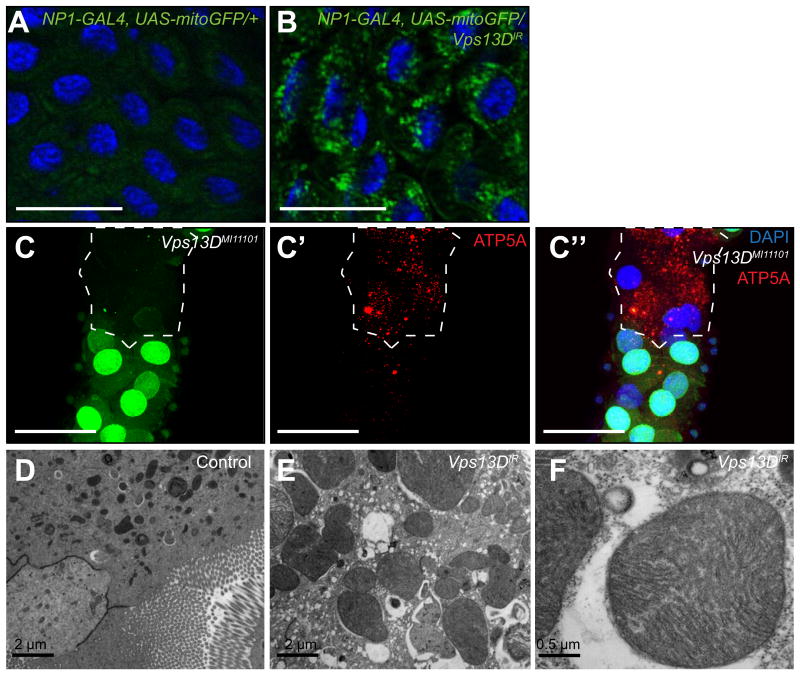
The functions of autophagy genes and Uba1 in the clearance of mitochondria [2] led us to consider if Vps13D is required for this process. Knockdown of Vps13D leads to a failure in mitochondrial clearance, as shown by a lack of mitochondrial targeted green fluorescent protein (mitoGFP) clearance compared to control midgut cells (Figure 2A and B). Consistent with these data, Vps13DMI11101 mutant midgut cells failed to clear mitochondrial ATP synthase (ATP5A) protein compared to neighboring control cells (Figure 2C-C”). The Vps13D mutant defect in the clearance of mitochondria prompted us to investigate the morphology of these cells in the midgut by transmission electron microscopy (TEM). Remarkably, Vps13D knockdown in midguts not only prevented clearance of mitochondria, but also resulted in a significant enlargement of mitochondria compared to those observed in control midguts (Figure 2D-F). In addition, a similar enlarged mitochondria phenotype was observed in Drosophila S2R+ cells following Vps13D knockdown (Figure S3D-E’). These data indicate that Vps13D is necessary for mitochondrial clearance in the Drosophila midgut, and that these mitochondria are enlarged.
Figure 2. Vpsl3D function is required for mitochondrial clearance and size control in the Drosophila intestine.
(A) Mito-GFP in control gut and (B) Vps13D knockdown midguts 2 h after puparium formation. Results are representative of at least three biological replicates. Scale bars represent 50 μm.
(C-C”) MiMIC insertion Vps13D mutant midgut cells (lacking GFP) possess persistent mitochondrial ATP5A protein compared to neighboring control cells (GFP-positive) indicating a defect in the clearance of mitochondria. Scale bars represent 50 μm.
(D-F) Knockdown of Vps13D results in enlarged midgut mitochondria compared to mitochondria from control w1118 animal midguts. Results are representative of at least three biological replicates.
See also: Figure S3
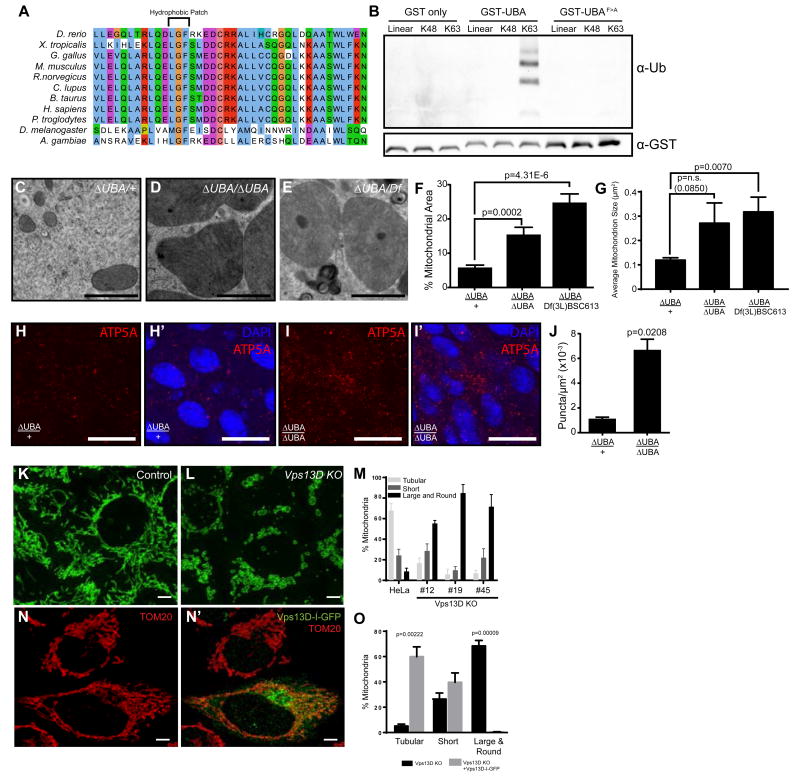
The putative ubiquitin binding UBA domain of Vps13D is conserved from flies to humans, and importantly, contains a conserved hydrophobic M/L-G-F/Y patch that is known to be necessary to bind ubiquitinated proteins [8, 9] (Figure 3A). In order to determine the importance of the Vps13D UBA domain, we first performed an in vitro binding assay to determine whether this domain binds ubiquitin. Using a GST-tagged Vps13D UBA domain, we determined that this domain was able to bind K63 tetra-ubiquitin chains, but neither to linear nor K48-linked tetra-ubiquitin chains (Figure 3B). By contrast, neither the mutant containing a phenylalanine to alanine mutation in the hydrophobic patch (UBAF>A-GST) nor a GST-alone control was able to bind any of the tetra-ubiquitin chains. The fact that the Vps13D UBA domain preferentially binds K63-linked and not K48-linked ubiquitin agrees with previous data indicating that cell size reduction is independent from proteasome activity [2], and supports the hypothesis that the Vps13D UBA domain and K63-linked ubiquitin is important for the mitochondrial phenotypes in animals with decreased Vps13D function.
Figure 3. Vps13D ubiquitin binding is required for mitochondrial size in Drosophila and humans.
(A) Vps13D contains a UBA domain with a conserved hydrophobic patch known to mediate the interaction with poly-ubiquitin.
(B) Interaction of the Vps13D UBA domain or a phenylalanine to alanine mutant with tetra-ubiquitin chains. Results are representative of at least three binding assays.
(C) TEM highlighting the mitochondria of Vps13D UBA domain (∆UBA) heterozygous and (D) homozygous mutants as well as (E) Vps13D∆UBA/Df(3L)BSC613 midguts. Scale bars represent 1 μm.
(F) Percent area occupied by mitochondria and (G) average mitochondrion size quantitated from at least 5 fields from 3 intestines per sample.
(H-H’) ATP5A staining in Vps13D∆UBA/+ control guts 2 h after puparium formation. Results are representative of six biological replicates. Scale bars represent 25 μm.
(I-I’) ATP5A staining in Vps13D∆UBA/Vps13D∆UBA mutant guts 2 h after puparium formation. Results are representative of six biological replicates. Scale bars represent 25 μm.
(J) ATP5A staining is quantitated from 3 intestines each from heterozygous control and mutant midguts.
(K) TOM20 labeling of control and (L) Vps13D KO HeLa cells. Scale bars represent 5 μm.
(M) Quantitation of percent mitochondria representing tubular, short, or large and round phenotypes in control HeLa or Vps13D KO cell lines #12, #19 and #45. Results are representative of three biological replicates. For tubular mitochondria, P values are all <0.0001 for #12, #19 and #45 vs control HeLa. For short mitochondria, P=0.85, 0.07, 0.98 respectively for #12, #19 and #45 vs control HeLa. For large and round mitochondria, P values are all <0.0001 for #12, #19 and #45 vs control HeLa.
(N-N’) Vps13D tagged internally with GFP (Vps13D-I-GFP) rescues the large and round mitochondrial phenotype seen in Vps13D KO cells. Scale bars represent 5 μm. Results are quantitated in (O) and are representative of three biological replicates.
See also: Figures S3 and S4
In order to further analyze the importance of the UBA domain in Vps13D phenotypes, we used CRISPR/Cas9 to create animals containing an in-frame deletion of the UBA domain. This mutant fly strain, Vps13D∆UBA, is 43.6% homozygous lethal (n = 172) indicating that the UBA domain is important for some of the functions of the protein and that some Vps13D function is retained compared to the 100% lethal Vps13DMI11101 mutant. Consistent with this conclusion, Vps13D∆UBA mutant cells possess Vps13D protein (Figure S3F). Significantly, homozygous Vps13D∆UBA mutant animal midgut cells possessed enlarged mitochondria (Figure 3C-G) and larger cell size (p=0.0145, data not shown), indicating that the UBA domain alone is necessary for these phenotypes. Notably, Vps13D∆UBA mutant animal midgut cells possessed persistent mitochondrial ATP5A protein compared to control midgut cells (Figure 3H-J), indicating that the UBA domain of Vps13D is required for mitochondrial clearance. TEM analyses of Vps13D∆UBA/Df(3L)BSC613 midguts indicated that these intestines also have enlarged mitochondria (Figure 3E and G), suggesting that the enlarged mitochondria seen in Vps13D∆UBA homozygous mutants are not likely due to an off-target effect of the CRISPR gRNAs.
Human VPS13D is 33% identical with Drosophila Vps13D, suggesting the possibility of a conserved function between these distant taxa. We investigated this possibility by using CRISPR/Cas9 to create multiple HeLa cell lines that are deficient in Vps13D function. While control HeLa cells possess mostly tubular mitochondria, three independent Vps13D knockout (KO) cell clones possessed significantly more large and rounded mitochondria (Figure 3K-M), consistent with the phenotype observed in the Drosophila intestine. Significantly, introduction of a Vps13D plasmid tagged internally with GFP rescued the large round mitochondrial phenotype (Figure 3N-O). These data indicate that Vps13D is required for proper mitochondrial morphology, and that this function is conserved between flies and humans. Like in the fly, deletion of the Vps13D UBA domain in HeLa cells (Vps13D KO#12) also resulted in significant mitochondrial morphology defects (Figure 3M), highlighting the importance of the ubiquitin binding domain for Vps13D's function across species. We also examined the effect of Vps13D knockout on mitophagy in HeLa cells. Although Vps13D knockout HeLa cells had decreased mitophagy as measured by the clearance of mitochondrial COXII protein compared with control HeLa cells, the impact of Vps13D was not as strong as was observed in Atg5 mutant HeLa cells (Figure S4A-B).
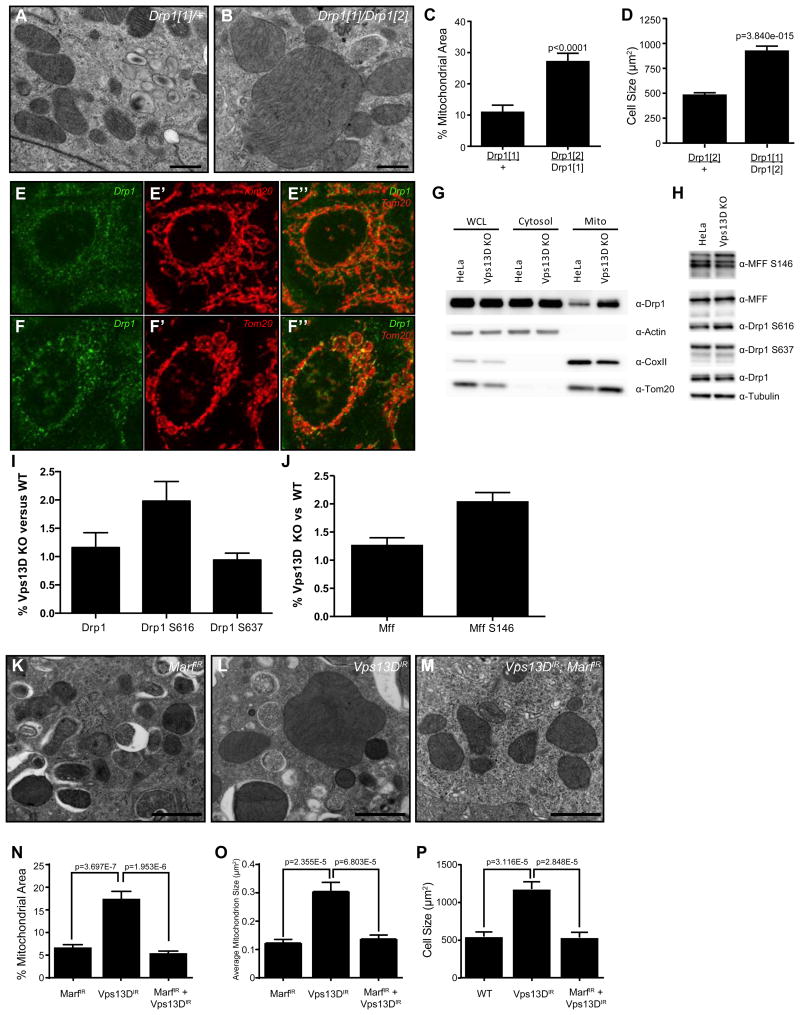
The cristae of enlarged Vps13D mitochondria are juxtaposed and appear intact (Figure 2F), suggesting that the enlargement of mitochondria might be due to altered dynamics, as opposed to swelling. Since blocking mitochondrial fission results in compensatory fusion [10], we examined the midgut cells of intestines isolated from animals that possess a mutation in the mitochondria fission gene Drp1 to determine whether their phenotype was similar to Vps13D mutant mitochondria. Not only did homozygous Drp1 mutants have enlarged mitochondria, but mutant midgut cells also exhibited a defect in mitochondrial clearance and cell size reduction compared to heterozygous Drp1/+ control midgut cells (Figure 4A-D). The similarity of Drp1 and Vps13D mutant phenotypes suggest that they have related functions in mitochondrial homeostasis and clearance. Drp1 encodes a GTPase that directly regulates mitochondrial fission [11], suggesting that Vps13D may influence Drp1 recruitment to mitochondria. We investigated this possibility in Vps13D mutant HeLa cells since antibodies do not exist against Drosophila Drp1. Not only did we observe recruitment of Drp1 to Vps13D mitochondria (Figure 4E-F”), but the amount of Drp1 associated with mitochondria appeared to be elevated compared to wild type HeLa cells (Figure 4G). Even with increased Drp1 recruitment, however, the Vps13D mitochondria appeared large and round (Figure 4F-F”). Furthermore, the amount of both phosphorylated Drp1 and phosphorylated mitochondrial fission factor (MFF) in Vps13D mutant HeLa cells was elevated compared to wild type HeLa cells (Figure 4H-J).
Figure 4. Decreased Vps13D function leads to enlarged mitochondria because of a defect in mitochondrial dynamics.
(A-B) TEM of Drp1 heterozygous (A) versus transheterozygous (B) mutant intestines. Scale bars represent 0.5 μm.
(C) Homozygous Drp1 mutant intestine cells fail to clear mitochondria compared to a control Drp1/+ intestine (results are quantitated from at least 4 fields from 2 (controls) or 3 (mutant) intestines).
(D) Drp1 mutant intestine cells exhibit a defect in programmed cell size reduction (quantitated from 41 cells from 4 guts/genotype).
(E-E”) Drp1 localization in control versus (F-F”) Vps13D KO HeLa cells co-stained with Tom20. Scale bars represent 5 μm.
(G) Western blot analysis of Drp1 accumulation on mitochondria in Vps13D KO versus wild type HeLa cells. Actin, COXII, and Tom20 were used to identify the cytosolic (cytosol) and mitochondrial (mito) fractions.
(H) Western blot analysis of Drp1 and MFF phosphorylation in Vps13D KO versus control HeLa cells. Western results are representative of at least three independent experiments.
(I, J) Quantitation of three replicates of the western blot in (H).
(K) TEM of Marf IR, Vps13D IR (L), and Vps13D, Marf double knockdown (M) intestines. Scale bars represent 1 μm.
(N) Percent mitochondrial area, (O) average mitochondrion size, and (P) cell size in Marf, Vps13D, and Marf Vps13D double knockdown intestines. Results are representative of at least three biological replicates.
See also: Figure S4
The enlarged mitochondrial phenotype in Vps13D mutant cells could suggest that mitochondria fission is reduced and that mitochondria fusion has occurred, indicating that the dynamics of this organelle are altered. If a defect in fission is driving fusion to result in enlarged mitochondria, then concomitantly blocking fusion should suppress the enlarged mitochondria phenotype. Therefore, we tested if decreased Vps13D function results in enhanced mitochondrial fusion in Drosophila midgut cells since Vps13D and fusion can be impaired simultaneously. Indeed, simultaneous knockdown of the mitochondrial regulator of fusion Marf (Mitofusin in mammals) [12] and Vps13D resulted in mitochondria similar to those observed in midguts with Marf knockdown alone, and rescued the Vps13D mitochondrial clearance and cell size defects (Figure 4K-P). This attenuation is not due to the presence of multiple UAS-responsive transgenes as knockdown of both Vps13D and GFP resulted in enlarged mitochondria (Figure S4C-F). Thus, it appears that reduced Vps13D function results in an impaired mitochondrial fission and, thus, a block in mitophagy. These data implicate Vps13D in mitochondrial fission downstream of Drp1 and upstream of the induction of autophagy.
Conclusion
We demonstrate that the Drosophila midgut is an excellent model for the in vivo study of mitophagy, and identify TSG101, Vps36 and Vps13D as genes that influence autophagy in these cells. Previous studies indicated that ESCRT components influence autophagy at a late stage in the process of autophagosome maturation [13]. By contrast, our results indicate that the ESCRT components TSG101 and Vps36 influence autophagy at a previously undescribed step upstream of Atg8a puncta formation. This phenotype is similar to what we observe in Vps13D mutant cells, and suggests a possible relationship between these ESCRT proteins and Vps13D. We conclude that Vps13D is necessary for cell size reduction, autophagy, and mitochondrial clearance in the midgut of Drosophila, and that lack of Vps13D leads to an apparent defect in mitochondrial dynamics, resulting in enlarged mitochondria. Significantly, Vps13D has a related function in mitochondrial homeostasis in human cells, both illustrating the value of the midgut model system and the importance of Vps13D in cell health. Additionally, we show that the Vps13D UBA domain binds K63-linked ubiquitin, and is necessary for the role of Vps13D in mitochondrial fission and clearance of mitochondria.
Mitochondrial fission has been linked to mitophagy [14], but how and why fission of mitochondria connects to the clearance of this important organelle is a mystery. It is possible that fission and clearance of a damaged portion of a mitochondrion involves a mechanism to distinguish damaged from functional mitochondrial compartments. Interestingly, Vps13D functions in a previously undescribed cellular space that links mitochondrial fission downstream of both phosphorylated Drp1 and MFF to influence autophagy. Consistent with our observations, yeast Vps13 has been recently implicated in inter-organelle contacts, including the vacuole, endoplasmic reticulum and mitochondria [15]. Therefore, it appears that Vps13D is a key regulator that unites mitochondrial fission and mitophagy, important processes that have been largely studied in isolation.
Significantly, our studies provide the first clear link between a ubiquitin binding protein and mitochondrial size. It is possible that the ubiquitin binding function of Vps13D in mitochondrial dynamics is unrelated to the role of Vps13D in autophagy. However, the lethal mutant phenotype of Vps13D appears to be stronger than Drp1 mutants, suggesting that Vps13D has unique properties beyond the regulation of fission and core fission machinery. As inhibition of mitochondrial fission is known to attenuate neurotoxicity in models of Parkinson's disease [16], it would be interesting to determine the role of Vps13D in such diseases.
Method Details
Induction of cell clones, mito-GFP experiments, and quantitation
To induce RNAi in clones of midgut or fat body cells, virgin females of y w hsFlp; pmCherry– Atg8a; Act >CD2 >GAL4, UAS–nlsGFP/TM6B flies were crossed to indicated RNAi lines. One-day egg lays were heat shocked at 37 °C for 15 minutes. To induce loss-of-function clones, we crossed y w hsFlp; CyO/Sp; FRT2A Ubi–nlsGFP virgin females with either MiCG32113MI11101 or Vps13D∆UBA recombined onto the FRT2A chromosome. One day egg lays were heat-shocked for 1 h at 37 °C. Mitochondrial targeted GFP (UAS-mito-GFP) was expressed in Drosophila intestine using the NP1-GAL4 driver and intestines were assessed for clearance of mitochondria 2h APF. For fat body experiments, wandering larvae were starved for 4 h in 20% sucrose (Fisher) before dissection. For staging midgut experiments, white prepupae were placed on wet filter paper for 2 h before dissection. Atg8a punctae were quantitated using ImageJ. Cell size was measured using the AxioVision software (Zeiss).
Assembly of a comprehensive list of candidate ubiquitin receptors in Drosophila
To assemble a comprehensive list of candidate Drosophila ubiquitin receptors, a combination of two approaches were used. First, generalized profiles [19] of all known ubiquitin-binding domains [20] were generated and used to search the Drosophila proteome [21]. Significant matches with p-values better than 0.01 were included in the list. To detect more divergent copies of the shorter ubiquitin binding domains, the HHsearch method [22] was used to search a proteome-wide collection of multiple alignments of Drosophila melanogaster proteins with orthologs from 14 insect species. HHsearch matches with p-values better than 0.001 were tested for their plausibility, and if successful, were added to the ubiquitin-binding domain encoding gene list. Alignment of Vps13D UBA domains done by Clustal Omega [23] and Jalview [24].
Electron Microscopy and Quantitation
Intestines were dissected in PBS (Gibco) 2 h after puparium formation and were fixed in a solution of 2.5% glutaraldehyde (Electron Microscopy Sciences) in 0.1M sodium cacodylate buffer, pH 7.4 (Electron Microscopy Sciences) for 30 minutes at room temperature. Following fixation, the guts were washed 4 times for 10 minutes in 0.1M sodium cacodylate buffer. Following post-fixation in 1% osmium tetroxide for 1 h at room temperature, the intestines were water washed and dehydrated through graded ethanols, treated with propylene oxide and infiltrated for embedding in SPI-pon/Araldite. Ultrathin sections were cut on a Reichert-Jung Ultracut E microtome and imaging was performed using a FEI Technai Spirit 12 TEM. Images were taken down the length of the gut to ensure an unbiased approach. Mitochondria were measured using the AxioVision software (Zeiss) and the percent mitochondrial area was calculated by dividing area of the mitochondria by the total gut area in each image. Average mitochondria size was calculated by determining the area of all mitochondria in each image and graphing the average from the indicated number of guts and images. All images were reviewed and representative images were chosen for analysis. P values were calculated using an unpaired T test with Welch's correction. Data is plotted as the mean +/- SEM.
Rapamycin and H2O2 treatment
Drosophila larvae were fed either rapamycin or H2O2 as previously described [25]. Early third-instar larvae were fed 20% sucrose in PBS for 45 min before placing them on food containing either 5 μM rapamycin (Sigma-Aldrich, St. Louis, MO, USA; from 1 mM in ethanol) or an equal amount of ethanol (control) for 4 h. For H2O2, early third-instar larvae were placed water-soaked filter paper for 45 min at 25°C, and then placed on food containing either 1.5% H2O2 (Fisher Scientific, Fair Lawn, NJ, USA) or an equivalent amount of water as a control for 7 h. The intestines were imaged using a Zeiss LSM 700 confocal microscope.
Immunolabeling and microscopy
Midguts were dissected in PBS, fixed with 4% paraformaldehyde in PBS, washed with PBS 0.1% Triton X-100 (PTX), and incubated with primary antibodies in PTX. For immunolabeling, we used mouse anti-ubiquitin (fk2; 1:100), anti-Vpsl3D (1:50, produced by Primm biotech), rabbit anti-ref(2)p (1:2000), mouse anti-ATP synthase complex V (1:1000) [2], and rat anti-Atg8a (1:300) [26]. For secondary antibodies, we used anti-rabbit, anti-mouse or anti-rat Alexa Fluor 488 or 546 antibody (1:250) and mounted samples in VectaShield containing DAPI (Vector Lab) to detect DNA. We imaged samples using a Zeiss LSM 700 confocal microscope. For mCherry-Atg8a imaging, we briefly fixed samples with 4% formaldehyde in PBS, mounted in VectaShield, and imaged samples with a Zeiss Axiophot II microscope. P values were calculated using an unpaired T test with Welch's correction. Data is plotted as the mean +/-SEM.
Cell line construction and cell culture
Drosophila S2R+ cells were stably transfected with pCasper-tub-mitoGFP encoding a tubulin promoter driven human COX VIII mitochondrial targeting signal fused to the N terminus of EGFP.
dsRNA for Vpsl3D RNAi treatment was produced and cells were treated and imaged as previously described [25]. dsRNA RNAi was produced by in vitro transcription of a polymerase chain reaction (PCR)-generated DNA template from Drosophila genomic DNA containing the T7 promoter sequence on both ends. Genomic DNA was harvested from Drosophila S2 cells using the Wizard Genomic DNA Purification Kit from Promega (Madison, WI, USA). 20 μg of dsRNA in water or an equal amount of water as control was added to triplicate wells in 6 well plates. S2R+ cells were resuspended in serum-free media at 0.5 x 106 cells/ml and 1 ml of this suspension was added as a control to wells containing dsRNAs. Cells were incubated at room temperature for 30 min prior to the addition of 3 ml of complete medium. Cells were incubated for 3 days at 25°C and imaged using an Olympus 1X71 inverted microscope (Olympus, Shinjuku, Tokyo, Japan). Images were obtained using a Qlmaging Retiga 1300 camera and processed using QCapture 2.99.5 (Qlmaging, Burnaby, BC, Canada).
HeLa cells were used because of their strength as a model for studies of mitochondrial dynamics and mitophagy in human cells. For HeLa Vpsl3D knockout cell line construction, CRISPR gRNAs were cloned into the pSpCas9(BB)-2A-GFP vector (Addgene Plasmid #48138) and transfected into HeLa cells with FuGene HD (Promega). Two days later, cells were FACS sorted and plated in 96-well plates. Single colonies were expanded and screened with PCR (see Table S2 for primers and gRNA sequences).
To make Vps13D-I-GFP (Vps13D tagged internally with GFP), the Vps13D 3′ ORF (sequence after exon40 to the stop codon) was PCR amplified and cut with NheI/SalI to clone into pEGFP-C1 at NheI/SalI by Gibson cloning (New England Biolabs). Next, XbaI, BsiWI, and AgeI sites were introduced and this modified pEGFP-Vps13D 3′ ORF was digested with XbaI/AgeI. GFP was then PCR amplified and cloned into these sites. The resulting construct was then digested with NheI/XbaI and the Vps13D 5′ ORF (sequence before exon 40) was PCR amplified and cloned into these sites using the Gibson kit. All PCR was conducted with Q5 DNA polymerase (New England Biolabs) and the final construct was confirmed by Sanger sequencing. Primer sequences are available upon request.
Protein expression and purification
The Drosophila Vps13D UBA domain and surrounding amino acids (KADSDLEKAAPLVAMGFEISDCLYAMQINNWRINDAAIWLSQQ as determined using Phyre2) [27], or the F>A mutant within this sequence (UBAF>A), were inserted into the pGEX-6P-2 GST expression vector (GE Healthcare Life Sciences). GST alone, GST-UBA, and GST-UBAF>A were expressed in E. coli BL21 and immobilized with Pierce Glutathione Magnetic beads in binding/wash buffer (125mM Tris, 150mM NaCl, pH 8.0).
In vitro ubiquitin binding
After immobilization onto glutathione magnetic beads, GST alone, GST-UBA, and GST-UBAF>A bead-bound proteins were washed with ubiquitin wash buffer (UWB; 150 mM NaCl, 50 mM Tris-HCl pH7.5, 0.1% NP-40, 5 mM DTT) and then incubated with 1 μg linear, K48, or K63 tetra-ubiquitin chains (Boston Biochem) in ubiquitin binding buffer (UBB; UWB with 0.5 mg/mL BSA) at 4°C for 16 h on a rotator. The beads were washed three times with UWB and were eluted with SDS-sample buffer. The samples were separated on a 4-20% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. Ubiquitin-HRP (Santa Cruz; 1:500) was used to probe for bound ubiquitin and GST-HRP (Thermo Fisher; 1:1000) was used to probe for GST.
Construction of CG32113 UBA domain mutant
Vps13D UBA domain mutant flies were generated using the CRISPR/Cas9 technique [28] . Two gRNAs were injected into vas-Cas9 flies by BestGene Inc. (Chino Hills, CA). Resulting flies were screened by PCR and direct sequencing of the target region. One offspring was found to carry an in-frame deletion of the Vps13D UBA domain region.
Western blot analysis
For analysis of mitophagy via western, we used Atg5 KO cells that were previously described [17]. Wild-type and knockout cells without or with stable expression of YFP-Parkin were seeded into 6- well tissue culture dishes and allowed to recover for 24 h. Cells were treated with 10uM oligomycin (Calbiochem), 4um antimycin A (Sigma) and 20uM QVD (ApexBio) in fresh growth media for times indicated. Cells were lysed in 1x LDS (Life Technologies) supplemented with 100uM dithiothreitol (Sigma) and heated for 10 minutes at 99°C. 35ug of cell lysate was electrophoresed on 4-12% NuPage Bis-Tris gels (Life Technologies) and transferred to polyvinyl difluoride membranes and immunoblotted as indicated. Actin (Cell Signaling), CoxII (Abcam) and MFNI antibodies were used for immunoblotting.
For analysis of Drp1 accumulation by immunofluorescence staining, cells were fixed with 4% paraformaldhyde in 1x PBS for 20 min at RT, blocked in 1x PBS with 0.15% Triton X-100 and 3% goat serum for 60 min, then incubated in primary antibody (in the same blocking solution) overnight at 4°C. Cells were then washed three times with 1x PBS containing 0.15% Triton X-100 at RT. Secondary antibody (in the same blocking solution) was added and incubated for 60 min at RT. Cells then washed three times and ready for confocal imaging. For quantitative counting, over 300 cells (100 cells each from three different fields) were counted. Experiments were repeated at least three times. The following antibodies were used: anti-Tom20 (Santa Cruz), anti-Drp1 (BD Bioscience), anti-COXII (Abcam), anti-Mff (Cell Signaling Technology), anti-phospho Mff S146 (Cell Signaling Technology), anti-phospho Drp1 S616 (Cell Signaling Technology), anti-phospho Drp1 S637 (Cell Signaling Technology), anti-Tubulin (Sigma) and anti-actin (Abcam).
Mitochondria Fractionation
Cells harvested from a 10-cm dish were washed twice with 5 ml 1xPBS and resuspended in 4.5 ml solution B (20 mM HEPES-KOH pH7.6, 220 mM Mannitol, 70 mM Sucrose, 1 mM EDTA, 0.5 mM PMSF), then transferred to a 15 ml glass homogenizer and homogenized with 30 strokes using a drill-fitted pestle. Cell homogenates were then spun at 800 g for 10 min at 4°C and the supernatant was collected and centrifuged at 10,000g for 20 min at 4°C. The supernatant (cytosolic fraction) was removed and the pellet (crude mitochondria) was resuspended in 200-500 μl of solution B. The cytosolic supernatant was concentrated with TCA and finally lysed in LDS loading buffer (Thermo Scientific). The crude mitochondria fraction was spun at 10,000 g for 5 min at 4°C again to remove any attached cytosolic contamination and the pellets were combined and resuspended in solution B and mixed with 2x LDS loading buffer.
Quantification and Statistical Analyses
All experiments were performed independently at least three times. Additional information on statistical details can be found in the figure legends. P values were calculated using a two-tailed unpaired t-test. For animal studies, sample sizes were determined empirically based on previous studies to ensure appropriate statistical power. No animals were excluded from statistical analyses, the experiments were not randomized, and the investigators were not blinded.
Table S1. List of ubiquitin binding protein-encoding genes screened. Related to Figures 1 and S2, STAR Methods.
Supplementary Material
Acknowledgments
We thank T. Fortier, G. Juhasz, S. Mamoor, P. Meier, N. Silverman, the Baehrecke laboratory, the Bloomington Stock Center, the Vienna Drosophila Resource Center, the Kyoto Drosophila Genetic Resource Center, and the Electron Microscopy Core Facility at UMass Medical School for flies, antibodies, advice, and technical support. This work was supported by R01GM111658 to EHB and S10RR027897 to the UMass Medical School Electron Microscopy Core. C.W., D.A.S, H.A.B and R.J.Y are supported in part by the Intramural Research Program, NINDS, NIH.
Footnotes
STAR Methods: Detailed methods are provided in the online version of this paper and include the following:
KEY RESOURCES TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
EXPERIMENTAL MODELS AND SUBJECT DETAILS: Drosophila strains and cell lines
METHOD DETAILS: Induction of cell clones, mito-GFP experiments, and quantitation
Assembly of a comprehensive list of candidate ubiquitin receptors in Drosophila
Electron Microscopy and Quantitation
Rapamycin and H2O2 treatment
Immunolabeling and microscopy
Cell line construction and cell culture
Protein expression and purification
In vitro ubiquitin binding
Construction of CG32113 UBA domain mutant
Western blot analysis
Mitochondria Fractionation
QUANTIFICATION AND STATISTICAL ANALYSES
Supplemental Information: Supplemental Information includes four figures and can be found online at.
Author Contributions: A.L.A., C.W., T.-K.C., D.A.S., R.J.Y. and E.H.B. designed the experiments. All experiments were performed by A.L.A., C.W., T.-K.C., and D.A.S. TEM by A.L.A. and C. M. P., list of putative ubiquitin binding protein encoding genes was produced by K.H., A.L.A. and E.H.B. wrote the manuscript and all authors commented on it.
Declaration of Interests: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nature cell biology. 2014;16:495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- 2.Chang TK, Shravage BV, Hayes SD, Powers CM, Simin RT, Wade Harper J, Baehrecke EH. Uba1 functions in Atg7- and Atg3-independent autophagy. Nature cell biology. 2013;15:1067–1078. doi: 10.1038/ncb2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rusten TE, Stenmark H. How do ESCRT proteins control autophagy? Journal of cell science. 2009;122:2179–2183. doi: 10.1242/jcs.050021. [DOI] [PubMed] [Google Scholar]
- 4.Venken KJ, Schulze KL, Haelterman NA, Pan H, He Y, Evans-Holm M, Carlson JW, Levis RW, Spradling AC, Hoskins RA, et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nature methods. 2011;8:737–743. doi: 10.1038/nmeth.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Developmental cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, Lander ES, Sabatini DM. Identification and characterization of essential genes in the human genome. Science. 2015;350:1096–1101. doi: 10.1126/science.aac7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomen VA, Majek P, Jae LT, Bigenzahn JW, Nieuwenhuis J, Staring J, Sacco R, van Diemen FR, Olk N, Stukalov A, et al. Gene essentiality and synthetic lethality in haploid human cells. Science. 2015;350:1092–1096. doi: 10.1126/science.aac7557. [DOI] [PubMed] [Google Scholar]
- 8.Mueller TD, Feigon J. Solution structures of UBA domains reveal a conserved hydrophobic surface for protein-protein interactions. Journal of molecular biology. 2002;319:1243–1255. doi: 10.1016/S0022-2836(02)00302-9. [DOI] [PubMed] [Google Scholar]
- 9.Ohno A, Jee J, Fujiwara K, Tenno T, Goda N, Tochio H, Kobayashi H, Hiroaki H, Shirakawa M. Structure of the UBA domain of Dsk2p in complex with ubiquitin molecular determinants for ubiquitin recognition. Structure. 2005;13:521–532. doi: 10.1016/j.str.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Scott I, Youle RJ. Mitochondrial fission and fusion. Essays in biochemistry. 2010;47:85–98. doi: 10.1042/bse0470085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Bliek AM, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harbor perspectives in biology. 2013;5 doi: 10.1101/cshperspect.a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwa JJ, Hiller MA, Fuller MT, Santel A. Differential expression of the Drosophila mitofusin genes fuzzy onions (fzo) and dmfn. Mechanisms of development. 2002;116:213–216. doi: 10.1016/s0925-4773(02)00141-7. [DOI] [PubMed] [Google Scholar]
- 13.Rusten TE, Vaccari T, Lindmo K, Rodahl LM, Nezis IP, Sem-Jacobsen C, Wendler F, Vincent JP, Brech A, Bilder D, et al. ESCRTs and Fab1 regulate distinct steps of autophagy. Current biology: CB. 2007;17:1817–1825. doi: 10.1016/j.cub.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Mao K, Wang K, Liu X, Klionsky DJ. The scaffold protein Atg11 recruits fission machinery to drive selective mitochondria degradation by autophagy. Developmental cell. 2013;26:9–18. doi: 10.1016/j.devcel.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John Peter AT, Herrmann B, Antunes D, Rapaport D, Dimmer KS, Kornmann B. Vps13-Mcp1 interact at vacuole-mitochondria interfaces and bypass ER-mitochondria contact sites. The Journal of cell biology. 2017;216:3219–3229. doi: 10.1083/jcb.201610055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rappold PM, Cui M, Grima JC, Fan RZ, de Mesy-Bentley KL, Chen L, Zhuang X, Bowers WJ, Tieu K. Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nature communications. 2014;5:5244. doi: 10.1038/ncomms6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denton D, Chang TK, Nicolson S, Shravage B, Simin R, Baehrecke EH, Kumar S. Relationship between growth arrest and autophagy in midgut programmed cell death in Drosophila. Cell death and differentiation. 2012;19:1299–1307. doi: 10.1038/cdd.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bucher P, Karplus K, Moeri N, Hofmann K. A flexible motif search technique based on generalized profiles. Computers & chemistry. 1996;20:3–23. doi: 10.1016/s0097-8485(96)80003-9. [DOI] [PubMed] [Google Scholar]
- 20.Vogt B, Hofmann K. Bioinformatical detection of recognition factors for ubiquitin and SUMO. Methods in molecular biology. 2012;832:249–261. doi: 10.1007/978-1-61779-474-2_18. [DOI] [PubMed] [Google Scholar]
- 21.dos Santos G, Schroeder AJ, Goodman JL, Strelets VB, Crosby MA, Thurmond J, Emmert DB, Gelbart WM, FlyBase C. FlyBase: introduction of the Drosophila melanogaster Release 6 reference genome assembly and large-scale migration of genome annotations. Nucleic acids research. 2015;43:D690–697. doi: 10.1093/nar/gku1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soding J. Protein homology detection by HMM-HMM comparison. Bioinformatics. 2005;21:951–960. doi: 10.1093/bioinformatics/bti125. [DOI] [PubMed] [Google Scholar]
- 23.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular systems biology. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anding AL, Baehrecke EH. Vps15 is required for stress induced and developmentally triggered autophagy and salivary gland protein secretion in Drosophila. Cell death and differentiation. 2015;22:457–464. doi: 10.1038/cdd.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takats S, Nagy P, Varga A, Pircs K, Karpati M, Varga K, Kovacs AL, Hegedus K, Juhasz G. Autophagosomal Syntaxin17-dependent lysosomal degradation maintains neuronal function in Drosophila. The Journal of cell biology. 2013;201:531–539. doi: 10.1083/jcb.201211160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nature protocols. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, O'Connor-Giles KM. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics. 2014;196:961–971. doi: 10.1534/genetics.113.160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.