Figure 2.
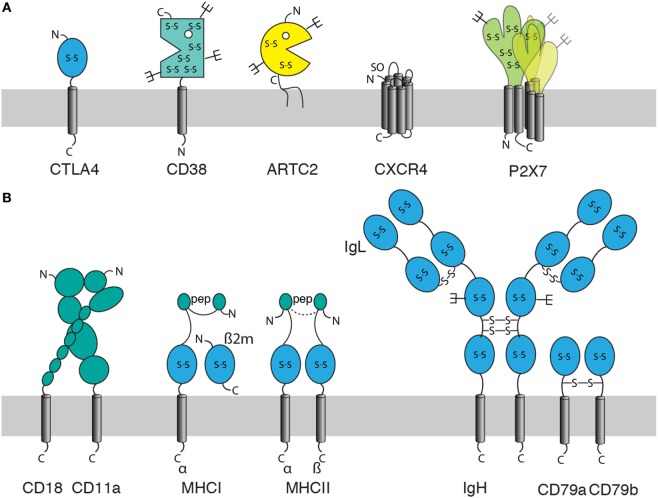
Features of membrane proteins to consider for generation of nanobodies (Nbs) by the cDNA immunization strategy. When using cDNA immunization as a strategy to induce a Nb response against a membrane protein, it is important to consider: the size and structure of its extracellular domain(s), the location of its N- and C-termini (out, in), posttranslational modifications [glycosylation, sulfation], and whether the protein can be expressed alone or in association with partner proteins only. Posttranslational modifications include glycosylation (forks), disulfide bridges (S-S), sulfation (SO), and loading with peptide (pep). (A) Examples of monomeric and homo-multimeric membrane proteins: GPI-anchored proteins such as ARTC2 consist of an extracellular domain covalently linked via the C-terminal amino acid to a membrane glycolipid. Single span membrane proteins possess extracellular and intracellular domains (or chains of linked domains). The extracellular domain is N-terminal in type I membrane proteins such as CTLA-4 and C-terminal in type II membrane proteins such as CD38. Most double-spanning (and tetra-spanning) membrane proteins have cytosolic N- and C-termini. Some double-spanning proteins such as P2X7 exist as homomultimers. Seven transmembrane proteins [G-protein-coupled receptors (GPCRs)] such as CXCR4 have an N-out C-in orientation and typically exist as monomers or dimers. (B) Effective expression of multimeric membrane proteins on the cell surface may require co-expression of one or more partners. These can be other transmembrane, secretory, or cytosolic proteins. Integrins such as LFA-1 (CD11a/CD18) are efficiently expressed on the cell surface only as a pair of non-covalently linked type I membrane proteins. MHC class I molecules are composed of a type I membrane protein, a non-covalently associated secretory protein (β2m) and a peptide docked in the peptide binding groove, MHC class II molecules are composed of two non-covalently linked type I proteins and a docked peptide. Many receptor complexes are assembled from three or more proteins, some of which may be linked by interchain disulfide bonds, as in the B cell receptor (BCR) complex where disulfide bridges link the two heavy chains (type I), each heavy chain to a light chain (secretory protein), and the two accessory type I proteins CD79a and CD79b.