Figure 4.
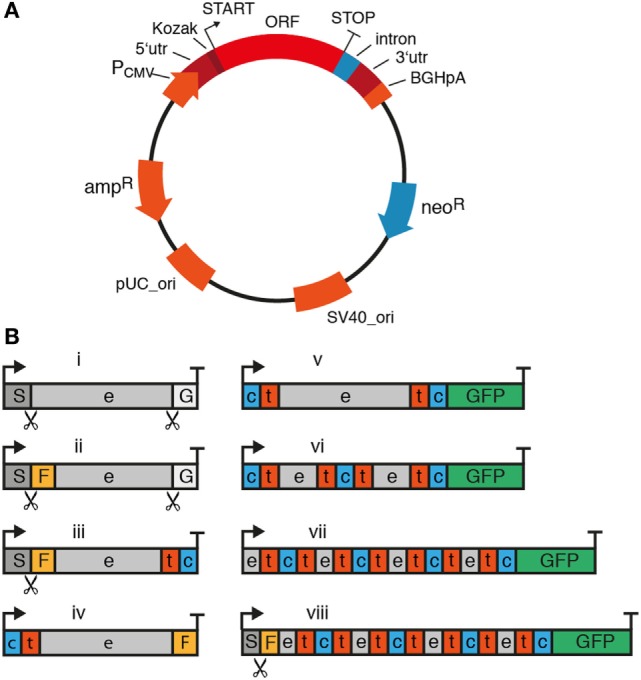
Essential and optional features of mammalian expression vectors for cDNA immunization of camelids. When using cDNA immunization as a strategy to induce a nanobody (Nb) response, it is important to consider components of the plasmid and the cDNA expression cassette. (A) Essential components of the plasmid are indicated in red and optional components in blue. The ampicillin resistance (ampR) and bacterial origin of replication (pUC_ori) are required for plasmid propagation in E. coli. The expression cassette for the membrane protein of interest contains the entire open reading frame (ORF) preceded by a strong viral promoter (e.g., the cytomegalovirus promoter, PCMV), a 5′ untranslated region (5′utr) and a Kozak sequence upstream of the start codon (arrow). The ORF is followed downstream of the stop codon by a 3′ untranslated region (3′utr) and a polyadenylation signal (e.g., of the bovine growth hormone, BGHpA). A mammalian antibiotic resistance gene (e.g., neomycin phosphotransferase, neoR) can be used for selecting stably transfected cells in culture. During cDNA immunization, this protein can help drive the immune response by providing peptides presented by MHC molecules. An intron can be placed anywhere between the start codon and the poly-adenylation signal to promote transcription and RNA processing. (B) In its simplest format (i), the expression cassette contains the full length open reading frame from start codon (arrow) to stop codon (T). A fused tag (e.g., Flag-tag, F) or fluorescent protein [e.g., green fluorescent protein (GFP)] can provide a means to verify expression of the protein at the plasma membrane. For fusion to a tag or protein, it is important to consider the availability and localization of the N- and C-termini of the membrane protein. Many membrane proteins contain a cleaved signal sequence (S) at the N-terminus, i.e., type I membrane proteins (iii) and some G-protein-coupled receptors (GPCRs) (viii). GPI-anchored proteins (i, ii) additionally contain a C-terminal signal sequence (G). The cleavage sites for these signal sequences are indicated by scissors. GFP usually works best when attached to a cytosolic terminus (v–viii). e: extracellular, t: transmembrane, c: cytosolic.
