Abstract
Background
Hyperthyroidism can complicate (mask) the diagnosis of chronic kidney disease (CKD) because it increases glomerular filtration rate and decreases body muscle mass, both of which can lower serum creatinine concentrations. Currently, there is no clinical test that can reliably predict which hyperthyroid cats have concurrent azotemic CKD that will become apparent after treatment of the hyperthyroidism.
Objectives
To investigate serum symmetric dimethylarginine (SDMA) concentration as a potential marker of masked azotemia in untreated hyperthyroid cats.
Animals
Two hundred and sixty‐two hyperthyroid cats and 206 aged‐matched, clinically normal cats.
Methods
Prospective study. We measured creatinine, urea nitrogen, SDMA, T4, and TSH concentrations before and 1, 3, and 6 months after treatment with radioiodine (131I) and classified 131I‐treated cats as azotemic or nonazotemic based on persistent, post‐treatment creatinine concentrations >2.1 mg/dL. Groups were compared via nonparametric tests, and diagnostic accuracy was determined by receiver operating characteristic analysis and logistic regression.
Results
No hyperthyroid cats were azotemic before treatment, but 42 (16%) became azotemic when rechecked at 4–8 months (median, 6 months) after 131I treatment; of these, 14 had high SDMA concentrations before treatment. As a diagnostic test for pre‐azotemic (masked) CKD in untreated hyperthyroid cats, SDMA showed a sensitivity of 33.3% and specificity of 97.7%.
Conclusions and Clinical Importance
Finding a high serum SDMA concentration in a hyperthyroid cat can help predict development of azotemia after treatment. The test has high diagnostic test specificity (few false‐positive results) but relatively low sensitivity (fails to predict azotemia in most hyperthyroid cats).
Keywords: Creatinine, Feline, Thyroid gland, Thyroxine, Thyroid‐stimulating hormone, Urine specific gravity
Abbreviations
- TSH
thyroid‐stimulating hormone
- T4
thyroxine
- CKD
chronic kidney disease
- SDMA
serum symmetric dimethylarginine
- USG
urine specific gravity
- IQR
interquartile range
- cTSH
canine TSH
Hyperthyroidism can complicate (mask) the diagnosis of concurrent chronic kidney disease (CKD) because it increases glomerular filtration rate (GFR)1, 2, 3, 4 and decreases body muscle mass,5 both of which lower serum creatinine concentrations. As a result, many hyperthyroid cats with concurrent CKD only develop azotemia after successful treatment when GFR and muscle mass return to euthyroid states.1, 2, 6, 7, 8, 9, 10, 11 Currently, no clinical test can reliably predict which hyperthyroid cats have concurrent, masked CKD.4, 9, 12 Identifying these cats could influence the choice of treatment for hyperthyroidism, as well as the care taken to minimize post‐treatment iatrogenic hypothyroidism, which can worsen existing azotemia and shorten survival time in cats with CKD.13, 14 Predicting which untreated hyperthyroid cats have masked concurrent CKD might also allow clinicians to intervene earlier in the course of the disease.
In cats, symmetric dimethylarginine (SDMA) has emerged as a potential serum biomarker for early detection of CKD with potential advantages over both urea nitrogen and creatinine.15, 16 Symmetric dimethylarginine is a byproduct of cellular protein metabolism, specifically the intranuclear methylation of l‐arginine residues; after proteolysis, these free SDMA residues are then released into the circulation.17 Symmetric dimethylarginine is not protein‐bound in plasma, is eliminated primarily via renal excretion (>90%), is freely filtered by the glomerulus, and is not secreted or re‐absorbed by the tubules.16, 18 In cats, SDMA concentrations correlate with GFR in an inverse linear relationship, with SDMA increasing as GFR decreases.16, 19 All of these factors make SDMA a useful marker for GFR.
Previous studies have demonstrated a strong relationship between serum SDMA and creatinine concentrations.15, 19, 20 Serum SDMA concentrations increase above normal when GFR decreases by approximately 40% below the normal rate,15 much earlier than the 75% decrease in GFR typically required for creatinine concentrations to increase above reference limits.21 Additionally, SDMA is not affected by changes in diet or body muscle mass,16, 22 unlike creatinine, which can be influenced by a number of extrarenal factors including diet or body muscle mass.23, 24 Therefore, SDMA concentrations might better reflect GFR in cats with increased protein intake or muscle wasting, both common findings in hyperthyroidism.5 This hypothesis is supported by data from IDEXX laboratories on 1,959 hyperthyroid cats, of which 3.5% had high serum creatinine concentrations but over 20% had high SDMA concentrations.25 Based on these IDEXX data, SDMA concentrations appear to detect masked CKD in hyperthyroid cats more accurately than creatinine concentrations. However, as this was a laboratory survey,25 no clinical information was reported regarding cats’ body weight, muscle condition, or treatment status. The influence of hyperthyroidism on SDMA metabolism in cats is not known, but in human patients with thyroid disease (without concurrent renal disease), increased SDMA concentrations are reported in both hyperthyroidism26 and hypothyroidism.27 This suggests that production or metabolism of SDMA might be altered by thyroid dysfunction independent of GFR.
To address these questions, we evaluated serum SDMA concentrations as a potential marker/predictor of masked azotemic CKD in hyperthyroid cats before 131I treatment and compared the diagnostic accuracy of SDMA for masked azotemia in these cats to that of other commonly used renal biomarkers (ie, serum creatinine and urea nitrogen concentrations; urine specific gravity). A secondary aim was to investigate the effects of successful 131I treatment on serum SDMA concentrations and to evaluate the influence of iatrogenic hypothyroidism on SDMA concentrations in these 131I‐treated cats.
Materials and Methods
Study Design and Selection of Animals
Hyperthyroid Cats Treated with 131I (n = 262)
All hyperthyroid cats referred to the Animal Endocrine Clinic (AEC) for treatment with 131I over the 21‐month period from July 2015 to April 2017 were evaluated for inclusion in this prospective before‐and‐after study. To be eligible for inclusion, untreated hyperthyroid cats underwent a thorough evaluation that included review of the past medical record, complete physical examination (including body weight, body and muscle condition scoring),5 routine laboratory testing (CBC and serum biochemical profile, including urea nitrogen and creatinine), determination of serum SDMA,16 determination of serum thyroid hormones (total T4 and thyroid‐stimulating hormone [TSH]),28, 29 and qualitative and quantitative thyroid scintigraphy.30, 31 In cats previously treated with methimazole, the drug was discontinued >1–2 weeks before evaluation. Finally, cats had to have successful resolution of the hyperthyroidism with the 131I treatment. For this study, we excluded hyperthyroid cats with pre‐existent azotemia (defined as serum creatinine >2.1 mg/dL), either when hyperthyroid or when euthyroid on methimazole treatment. We also excluded cats whose owners did not consent to return for follow‐up evaluation (Fig 1).
Figure 1.
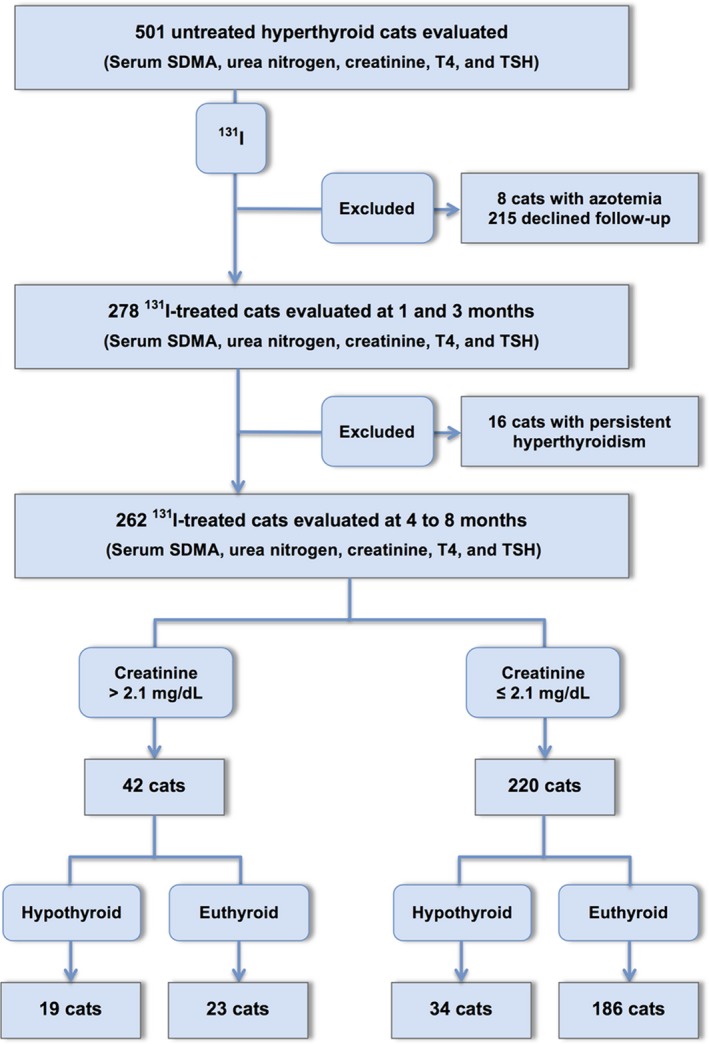
Flowchart for enrollment of hyperthyroid cats into study.
Two hundred and seventy‐eight 131I‐treated cats were initially enrolled into this study. We subsequently excluded 16 cats that remained hyperthyroid 1 and 3 months after 131I treatment (Fig 1). The remaining 262 cats were reexamined for this study at a median time of 6 months (4–8 months) after 131I treatment. At reevaluation, cats were weighed and had body condition and muscle condition scores assessed. Blood was collected for by measurement of serum biomarkers of renal function (creatinine, urea nitrogen, and SDMA concentrations) and thyroid function (T4 and TSH concentrations).
Based on results of follow‐up testing, we classified the cats as azotemic or nonazotemic. Renal azotemia was defined as a serum creatinine concentration >2.1 mg/dL in conjunction with inadequate urine concentrating ability (urine specific gravity [USG] <1.035) or persistent azotemia on 2 or more consecutive occasions (at 1‐ to 3‐month intervals) without evidence of a prerenal cause. Treated hyperthyroid cats that developed azotemia within the follow‐up period were defined as pre‐azotemic and were presumed to have had concurrent, but masked, azotemic CKD at the time of diagnosis. All other cats were defined as nonazotemic (serum creatinine concentration ≤2.1 mg/dL). We also classified the cats’ thyroid status as euthyroid (both serum T4 and TSH concentrations within reference interval), overtly hypothyroid (low serum T4 with high TSH concentration), or subclinically hypothyroid (low‐normal serum T4 with high serum TSH concentrations), as previously defined (Fig 1).28, 32
Clinically Normal, Euthyroid Cats (n = 206)
These cats were recruited as controls to establish reference intervals for serum urea nitrogen, creatinine, and SDMA concentrations. To be enrolled in this study, the cats had to be >7 years of age and were considered healthy based on an unremarkable client history, physical examination (ie, none had palpable thyroid nodules or small palpable kidneys), complete blood count, serum chemistry profile, urinalysis, and serum thyroid panel (serum T4 and TSH).
Assays for Serum Thyroid Hormone, Urea Nitrogen, Creatinine, and SDMA Concentrations
Serum SDMA concentrations were determined with an automatized biochemistry analyzer1 by a high‐throughput, competitive homogeneous immunoassay with a glucose‐6‐phosphate dehydrogenase conjugate and anti‐SDMA monoclonal antibody, validated as previously described.16 , 2,3 Serum urea nitrogen was measured by an enzymatic method, and serum creatinine was measured by a modified Jaffe method,33 both with an automatized biochemistry analyzer.1 Serum T4 and TSH were determined by assays validated for use in cats, as previously described.28
All blood samples were centrifuged within 1 hour after collection; serum was separated and stored at 4°C until assayed for SDMA, urea nitrogen, creatinine, T4, and TSH by a commercial laboratory4 the following day.
Data and Statistical Analyses
Data were assessed for normality by the D'Agostino‐Pearson test and by visual inspection of graphical plots.34 Data were not normally distributed; therefore, all analyses used nonparametric tests.35 Results are reported as median (interquartile range [IQR], 25th–75th percentile) and are represented graphically as box‐and‐whisker plots. For all analyses, statistical significance was defined as P ≤ 0.05. All statistical analyses were performed by proprietary statistical software.5,6
We used data from the 206 clinically normal cats to establish reference intervals for serum concentrations of urea nitrogen, creatinine, and SDMA by a nonparametric method to identify the central 95th percentile interval (ie, 2.5 through 97.5th percentile range).36 We also calculated 90% confidence intervals for the lower and upper limits of each reference interval (Table 1).
Table 1.
Reference intervals for serum concentrations of urea nitrogen, creatinine, and symmetric dimethylarginine (SDMA) based on results from 206 clinically normal, older cats. The 90% confidence intervals (CI) are calculated for both the lower and upper limits of each reference interval
| Analyte | Lower Reference Limit | 90% CI | Upper Reference Limit | 90% CI |
|---|---|---|---|---|
| Urea nitrogen (mg/dL) | 17 | 15–18 | 39 | 37–39 |
| Creatinine (mg/dL) | 0.8 | 0.8–0.9 | 2.1 | 2.0–2.1 |
| SDMA (μg/dL) | 5 | 3–6 | 14 | 14–15 |
Continuous variables (eg, serum urea nitrogen, creatinine, and SDMA concentrations) were compared between groups by the Kruskal‐Wallis test, followed by the Dunn's multiple comparisons test.37 Comparisons between 2 measurements between groups or within groups (before‐after) were analyzed with the Mann‐Whitney test and Wilcoxon signed ranks test, respectively. The Chi‐square test was used to determine differences in prevalence of muscle wasting between pre‐azotemic and azotemic groups of hyperthyroid cats, as well as to determine differences in prevalence of muscle wasting before and after 131I treatment. Correlation testing was performed with the Spearman rank correlation coefficient.
We then calculated the sensitivity and specificity for each renal biomarker (serum creatinine, urea nitrogen, SDMA, and USG) as a predictor of pre‐azotemic CKD with both the upper reference threshold value for each variable and the value identified by receiver operating characteristic (ROC) analysis.38, 39 Finally, we used multivariable logistic regression,40 with “azotemic” versus “nonazotemic” as the outcome variable and pretreatment body weight, body condition score, muscle condition score, age, T4 concentration, urea nitrogen, creatinine, USG, and SDMA, as predictor variables in a stepwise approach, to determine which variables independently helped predict development of azotemia after successful 131I treatment.
Results
Cats
All Cats with Hyperthyroidism
The 262 hyperthyroid cats ranged in age from 6 to 18 years (median, 12.0 years; IQR, 10–14 years). Breeds included domestic longhair and shorthair (n = 236), Maine Coon (n = 7), Siamese (n = 6), Norwegian Forest Cat (n = 3), Persian (n = 2), Russian Blue (n = 2), American Curl, Bombay, Burmese, Devon Rex, Oriental Shorthair, and Scottish Fold (1 cat each). Of these, 149 were female (56.8%) and 113 were male; all had been neutered.
Before treatment for hyperthyroidism, these cats had a median serum T4 concentration of 8.5 μg/dL (IQR, 6.4–11.4 μg/dL; reference interval, 0.9–3.9 μg/dL); thyroid scintigraphy confirmed bilateral thyroid disease in 157 cats (60%) and unilateral disease in 105 cats.
Cats received a median 131I dose of 1.9 mCi (IQR, 1.7–2.2 mCi; range, 1.2–13.9 mCi). At time of reevaluation (median, 6.0 months; IQR, 6–7 months), body weight had increased (median pretreatment, 4.5 kg [IQR, 3.7–5.4 kg]; median post‐treatment, 4.95 kg [IQR, 4.1–5.9 kg]; P < 0.001). After treatment, muscle condition improved, with the number of cats with muscle wasting decreasing from 180 (68.7%) to 107 (40.8%) cats (P < 0.001).
Hyperthyroid Cats Divided into Pre‐Azotemic Group and Nonazotemic Groups
Of the 262 hyperthyroid cats in this study, 42 (16%) developed azotemia after successful 131I treatment (pre‐azotemic group), whereas 220 cats remained nonazotemic (Fig 1). Cats with pre‐azotemic (masked) CKD did not differ from the cats that remained nonazotemic in breed or sex distribution, pretreatment body weight, or serum T4 concentrations (Table 2). However, hyperthyroid cats with pre‐azotemic (masked) CKD were older than cats that remained nonazotemic and had a higher prevalence of muscle wasting (Table 2).
Table 2.
Selected baseline clinicopathologic data (median; interquartile range) in 262 untreated hyperthyroid cats, divided into 2 groups: Pre‐azotemic cats, which subsequently developed azotemic chronic kidney disease after 131I treatment (n = 42), and Nonazotemic cats, which maintained normal serum creatinine concentrations (n = 220)
| Variable | Pre‐Azotemic (42) | Nonazotemic (220) | P Value |
|---|---|---|---|
| Age (years) | 14 (12–15) | 12 (10–13) | <0.001 |
| Body weight (kg) | 3.95 (3.4–5.2) | 4.6 (3.8–5.4) | 0.014 |
| Muscle wasting (%) | 36 (85.7) | 144 (65.5) | 0.010 |
| Serum urea nitrogen (mg/dL) | 35 (31–39) | 24 (21–28) | <0.001 |
| Serum creatinine (mg/dL) | 1.55 (1.4–1.8) | 1.0 (0.8–1.3) | <0.001 |
| Serum symmetric dimethylarginine (μg/dL) | 13.5 (11–16) | 9 (7–10) | <0.001 |
| Urine specific gravity | 1.019 (1.017–1.024)a | 1.042 (1.031–1.053)b | <0.001 |
| Serum T4 (μg/dL) | 8.0 (5.7–10.6) | 8.7 (6.5–11.7) | 0.12 |
Urinalysis performed in 40 of the 42 pre‐azotemic cats.
Urinalysis performed in 176 of the 220 nonazotemic cats.
Clinically Normal Cats
The 207 healthy cats ranged in age from 7 to 19 years (median, 11.0 years; IQR, 9–13 years). Breeds included domestic longhair and shorthair (185 cats), Siamese (5 cats), American Shorthair (4 cats), Chartreux (2 cats), Maine Coon (2 cats), Norwegian Forest Cat (n = 2), Persian (n = 2), Russian Blue (n = 2), Burmese, and Manx (1 cat each). Of these cats, 115 (55.8%) were female and 91 were male; all had been neutered. These cats did not differ from the hyperthyroid cats in breed or sex distribution. However, despite an identical age range, normal cats were younger than either the pre‐azotemic or nonazotemic hyperthyroid cats (P < 0.001).
Serum Biomarkers for Renal Disease, Before and After 131I Treatment
Untreated hyperthyroid cats had lower serum concentrations of creatinine and SDMA than clinically normal cats; urea nitrogen concentrations did not differ between hyperthyroid and clinically normal cats (Fig 2). Serum concentrations of urea nitrogen, creatinine, and SDMA all increased in hyperthyroid cats after 131I treatment, and post‐131I concentrations were all higher than concentrations in the clinically normal cats (Fig 2).
Figure 2.
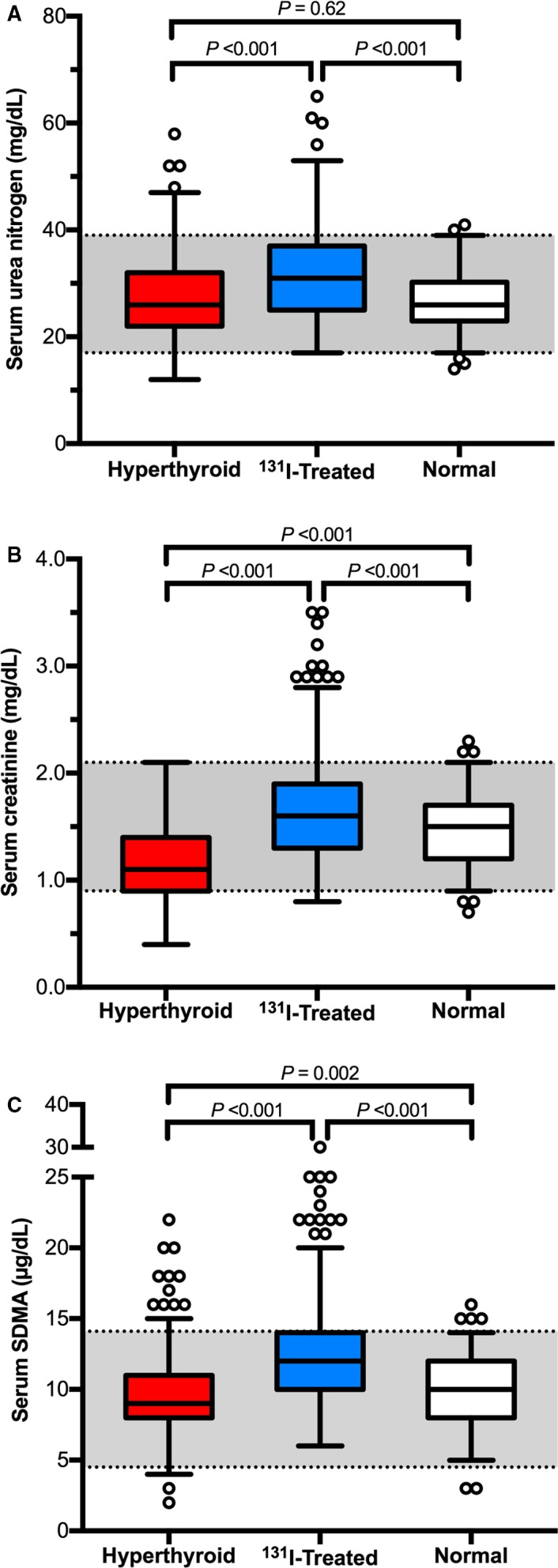
Box plots of serum concentrations of renal biomarkers in 262 hyperthyroid cats (before and after 131I treatment) and in 206 clinically normal cats. (A) Urea nitrogen; (B) creatinine; and (C) symmetric dimethylarginine. For each serum biomarker, significant differences between the 3 groups of cats are indicated. Boxes represent the interquartile range from the 25th to 75th percentile. The horizontal bar in each box represents the median value. The T‐bars represent the main body of data, which in most instances is equal to the range. Open circles represent outlying data points.
Pretreatment serum creatinine, urea nitrogen, and SDMA concentrations were all higher, and USG was lower in the 42 cats with masked azotemia, compared with the 220 cats that remained nonazotemic after 131I treatment (Table 2, Fig 3A–C); these differences persisted after 131I treatment (Table 3, Fig 3A–C). The cats that developed azotemia after 131I treatment were older and had lower body weights, as well as a higher prevalence of persistent muscle wasting, than did the cats that remained nonazotemic cats after treatment (Table 3).
Figure 3.
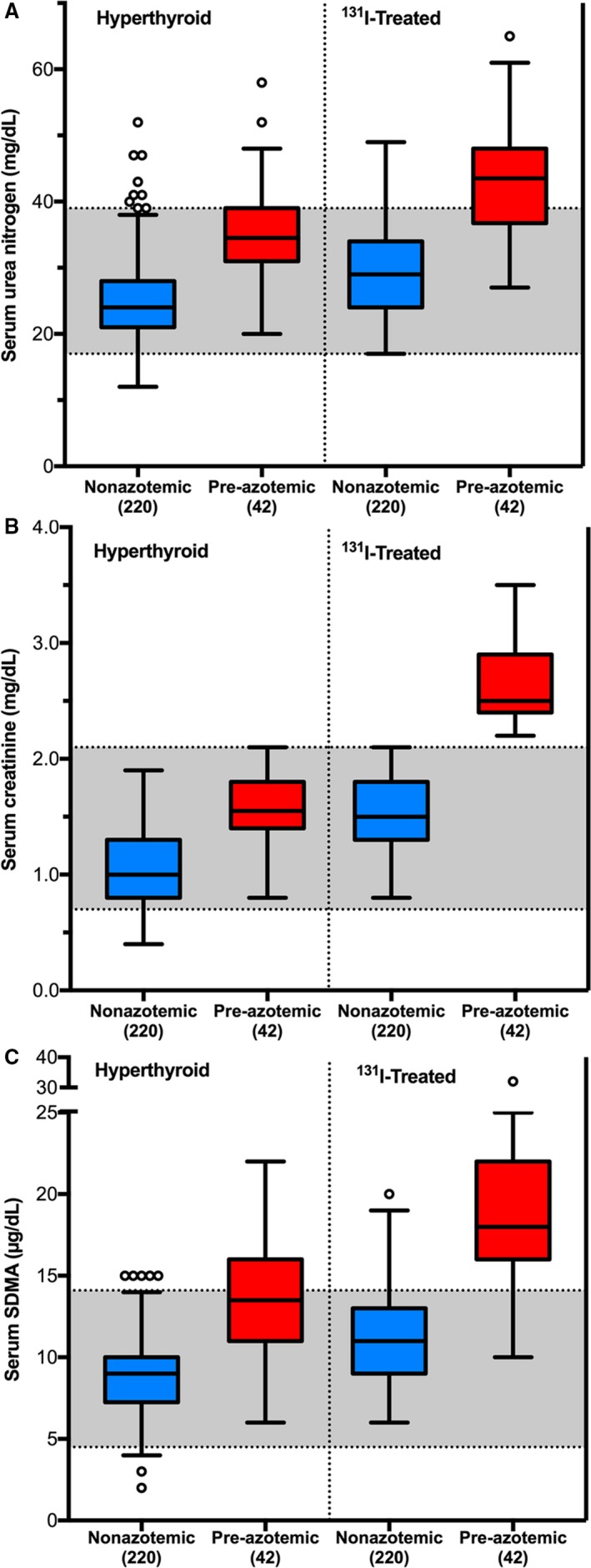
Box plots of serum concentrations of renal biomarkers in 262 hyperthyroid cats (before and after 131I treatment), divided into cats that developed azotemia and cats that remained nonazotemic after treatment. (A) Urea nitrogen; (B) creatinine; and (C) symmetric dimethylarginine.
Table 3.
Selected follow‐up clinicopathologic data (median; interquartile range) in 262 131I‐treated hyperthyroid cats, divided into 2 groups based on serum creatinine concentration: Pre‐azotemic cats, which have developed azotemic chronic kidney disease after 131I treatment (n = 42), and nonazotemic cats, which maintained normal serum creatinine concentrations after 131I treatment (n = 220)
| Variable | Pre‐azotemic (42) | Nonazotemic (220) | P Value |
|---|---|---|---|
| Age (years) | 14 (12–15) | 12 (10–13) | <0.001 |
| Body weight (kg) | 4.6 (3.8–5.5) | 4.9 (4.2–6.1) | 0.050 |
| Muscle wasting (%) | 24 (57.1) | 83 (37.7) | 0.025 |
| Serum urea nitrogen (mg/dL) | 44 (37–48) | 29 (24–34) | <0.001 |
| Serum creatinine (mg/dL) | 2.5 (2.4–2.9) | 1.5 (1.3–1.8) | <0.001 |
| Serum symmetric dimethylarginine (μg/dL) | 18 (16–22) | 11 (9–13) | <0.001 |
| Serum T4 (μg/dL) | 1.6 (1.2–2.1) | 1.9 (1.5–2.2) | 0.007 |
| Serum thyroid‐stimulating hormone (ng/mL) | 0.27 (0.08–0.89) | 0.10 (0.02–0.24) | <0.001 |
| Recheck time (months) | 6 (5–7) | 6 (6–7) | 0.16 |
Serum SDMA and creatinine concentrations showed a weak correlation in hyperthyroid cats before 131I treatment (r = 0.325, P < 0.001), with 19 of 262 (7.3%) cats having a high serum SDMA concentration (Fig 4A). Of these, 14 cats developed azotemic CKD after 131I treatment, whereas the other 5 remained nonazotemic after 131I treatment. In these 5 nonazotemic cats, high pretreatment SDMA concentrations normalized in 3 and increased slightly in 2 cats after 131I treatment.
Figure 4.
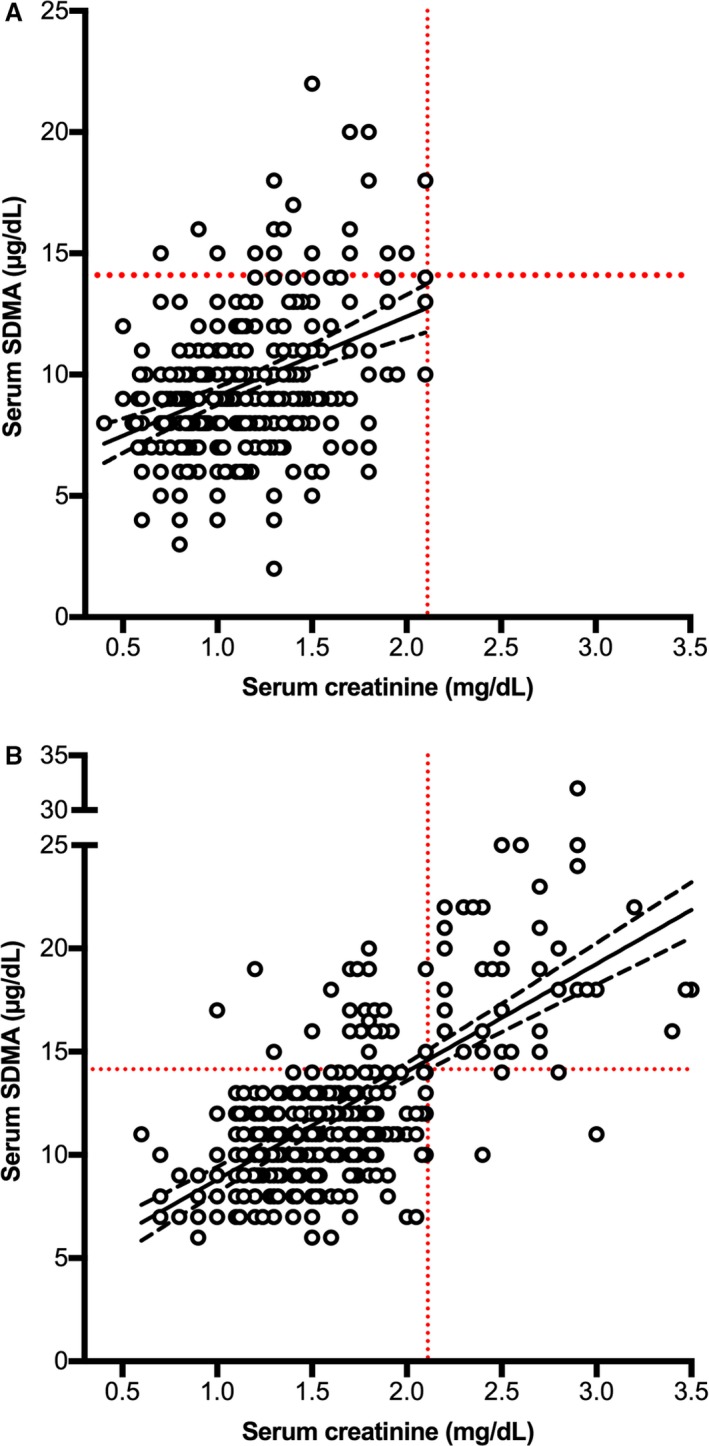
Scatter plots comparing serum creatinine and symmetric dimethylarginine concentrations in 262 cats before (A) and after treatment (B) with 131I. The solid line represents the line of best fit, and the dashed lines represent the 95% confidence intervals.
Serum SDMA and creatinine concentrations showed a stronger correlation in hyperthyroid cats after 131I treatment than before treatment (r = 0.664, P < 0.001), with 60 of 262 (22.9%) cats having a high SDMA concentration (Fig 4B). Of these, 38 (63%) had developed azotemic CKD, whereas 22 remained nonazotemic (Fig 4B).
Serum T4 and creatinine concentrations showed a modest negative correlation in hyperthyroid cats before 131I treatment (r = −0.40; P < 0.001); serum T4 and SDMA concentrations showed a very weak correlation in hyperthyroid cats before 131I treatment (r = −0.12; P = 0.049). After 131I treatment, serum T4 concentrations correlated negatively with both serum creatinine (r = −0.28; P < 0.001) and SDMA concentration (r = −0.22; P = 0.0004) to a similar extent.
Sensitivity and Specificity of Renal Biomarkers in Hyperthyroid Cats
Only 14 of 42 cats that developed azotemic CKD after treatment had a high pretreatment SDMA concentration (>14 μg/dL), with a test sensitivity of SDMA as a predictor of masked azotemia of only 33.3%, but a specificity of 97.7% (Table 4). Although the SDMA concentration was insensitive, it had higher test sensitivity than that of serum creatinine, even when the cutoff value for creatinine concentration was set well below the upper limit of the reference interval (Table 4). Lowering the upper cutoff for serum SDMA concentration from >14 μg/dL to >12 μg/dL resulted in almost a 2‐fold increase in test sensitivity with only a slight decrease in specificity.
Table 4.
Ability of pretreatment (hyperthyroid) serum symmetric dimethylarginine (SDMA) and creatinine concentrations and urine specific gravity (USG) to predict post‐131I azotemia
| Variable | Sample Size | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|
| SDMA >14 μg/dL | 262 | 33.3 (20–50) | 97.7 (98–99) |
| SDMA >12 μg/dL | 262 | 64.3 (48–78) | 93.2 (89–96) |
| SDMA >10 μg/dL | 262 | 78.6 (63–90) | 80.5 (75–86) |
| Creatinine >1.9 mg/dL | 262 | 11.9 (4–26) | 100 (98–100) |
| Creatinine >1.7 mg/dL | 262 | 33.3 (20–50) | 98.6 (96–100) |
| Creatinine >1.5 mg/dL | 262 | 50.0 (34–66) | 95.9 (92–98) |
| Creatinine >1.3 mg/dL | 262 | 78.6 (63–90) | 84.4 (79–89) |
| USG <1.035 | 215 | 92.1 (79–98) | 71.0 (64–78) |
| USG <1.030 | 215 | 89.7 (76–97) | 77.8 (71–84) |
| USG <1.025 | 215 | 79.5 (64–91) | 84.7 (79–90) |
Pretreatment USG (<1.035) had the highest test sensitivity (92%) as a predictor of masked azotemia of any of the renal analytes, but with a lower specificity (71%) than either SDMA or creatinine concentrations (Table 4).
Receiver operating characteristic analysis identified a creatinine concentration >1.3 mg/dL or an SDMA concentration >10 μg/dL as the best predictors of post‐treatment azotemia, although both of these cutoffs were within the reference intervals for these analytes.
Logistic regression identified pretreatment creatinine concentration, SMDA concentration, and USG (as a dichotomous variable with a threshold of 1.035) as independent predictors of post‐treatment azotemia according to the following equation:
Therefore, for a cat with a creatinine of 1.2 mg/dL, an SDMA of 14 μg/dL, and a USG <1.035, the Logit(P) would be 0.264, equal to a probability of 57% of developing post‐treatment azotemia. With an SDMA of 15 μg/dL, the probability would be 67% (Logit(P) = 0.724). Conversely, a cat with a creatinine of 1.2 mg/dL and an SDMA of 14 μg/dL but a USG >1.035 would have a 10% probability of developing post‐treatment azotemia (Logit(P) = −2.16).
Effect of Post‐Treatment Thyroid Status on Serum Biomarkers for Renal Disease
Of the 262 131I‐treated hyperthyroid cats, 209 (79.8%) became euthyroid (serum T4 and TSH concentrations both within the reference interval), whereas 53 (20.2%) developed iatrogenic hypothyroidism. Only 5 of the 53 cats had overt hypothyroidism (low T4 with high TSH concentration), whereas the remaining 48 cats had subclinical hypothyroidism (low‐normal T4 and high TSH concentrations) (Fig 1).
Iatrogenic hypothyroid cats had higher post‐treatment serum concentrations of creatinine, urea nitrogen, and SDMA concentrations than cats made euthyroid (Table 5). Iatrogenic hypothyroid cats (both overt and subclinical subtypes combined) had a higher prevalence of azotemia than cats made euthyroid (Table 5), but they did not differ from euthyroid cats in age, body weight, or prevalence of persistent muscle wasting (Table 5). Of all 53 hypothyroid cats, cats with overt hypothyroidism had a higher prevalence of azotemia than did cats with subclinical hypothyroidism (4/5 [80%] versus 15/48 [31%]; P = 0.031).
Table 5.
Selected baseline clinicopathologic data (median; interquartile range) in 262 131I‐treated hyperthyroid cats, divided into 2 groups based on thyroid status: Hypothyroid cats, which have developed high serum thyroid‐stimulating hormone (TSH) concentration after 131I treatment (n = 53), and euthyroid cats, which maintained normal serum TSH concentrations after 131I treatment (n = 209)
| Variable | Hypothyroid (53) | Euthyroid (209) | P Value |
|---|---|---|---|
| Age (years) | 13 (10–14.5) | 12 (10–14) | 0.28 |
| Body weight (kg) | 4.8 (3.8–5.8) | 4.9 (4.2–6.0) | 0.24 |
| Muscle wasting (%) | 26 (49.1) | 85 (40.7) | 0.28 |
| Serum urea nitrogen (mg/dL) | 33 (29–40) | 30 (25–37) | 0.004 |
| Serum creatinine (mg/dL) | 1.8 (1.5–2.5) | 1.6 (1.3–1.8) | <0.001 |
| Serum symmetric dimethylarginine (μg/dL) | 13 (11–16) | 11 (9–13) | <0.001 |
| Azotemia (%) | 19/53 (35.9) | 23/209 (11.0) | <0.001 |
| Serum T4 (μg/dL) | 1.4 (1.2–1.8) | 1.9 (1.6–2.3) | <0.001 |
| Serum TSH (ng/mL) | 0.73 (0.56–2.8) | 0.08 (0.02–0.17) | <0.001 |
| Recheck time (months) | 6 (6–7) | 7 (6–7) | 0.43 |
Discussion
The results of our study indicate that a high serum SDMA concentration (>14 μg/dL) in an untreated hyperthyroid cat is a highly specific but insensitive test for predicting post‐treatment CKD. In our study, a high SDMA identified only a third of cats that became azotemic after treatment. Although the sensitivity of serum SDMA concentration was far from perfect, it was a much better predictor of masked azotemia than the serum creatinine concentration. Conversely, we found that a pretreatment USG <1.035 was fairly sensitive and moderately specific for predicting azotemia. Combining results of diagnostic tests did not dramatically improve overall predictive performance of these analytes. However, by logistic regression, we found that all 3 variables (SDMA, creatinine, and USG) were independent predictors of post‐treatment azotemia, and we created a model to estimate probability of a cat developing post‐treatment azotemia with these variables. Finally, our results indicate that cats that became hypothyroid after 131I treatment are more likely to become azotemic than cats made euthyroid.
Serum SDMA concentrations were in the lower half of the reference interval (≤10 μg/dL) in 71% of these untreated hyperthyroid cats, likely due to the increased GFR associated with the hyperthyroid state. When cats with pre‐azotemic CKD were excluded, nonazotemic cats had even a greater proportion (80%) of serum SDMA concentrations ≤10 μg/dL, and over 90% of these cats had SDMA concentrations ≤12 μg/dL. For use as a diagnostic test for predicting masked azotemia in a untreated hyperthyroid cat, lowering the SDMA cutoff to >12 μg/dL or >10 μg/dL (below the reference limit of 14 μg/dL) increased the test sensitivity to 64% and 79%, respectively, while maintaining reasonable specificity (Table 4). Similarly, lowering the cutoff for serum creatinine to 1.3 mg/dL or USG to >1.030 provided the optimal points to distinguish the pre‐azotemia and nonazotemic groups of cats (Table 4).
We found a weak, negative relationship between serum T4 and SDMA concentrations in these hyperthyroid cats before 131I treatment. This is unlike the results reported in human patients with hyperthyroidism, in whom investigators found a strong positive correlation between SDMA and hyperthyroid status.41 This suggests that changes in SDMA concentrations in these untreated hyperthyroid cats primarily reflect changes in glomerular filtration rather than a direct effect of hyperthyroidism per se, but further studies on the metabolism of SDMA in hyperthyroid cats are warranted.
In previous studies of cats, pretreatment values for serum creatinine, urea nitrogen, or urine specific gravity have not been proven reliable in predicting development of azotemia after treatment of hyperthyroidism.1, 6, 9 Some studies suggested that direct measurement of GFR might be useful,3, 8 but other studies report that measurement of GFR alone is not a reliable predictor due to significant overlap in values between pre‐azotemic and nonazotemic cats.1, 2 In our study, cats that developed azotemia after 131I treatment were older and had higher pretreatment concentrations of creatinine and urea nitrogen concentrations compared with the cats that remained nonazotemic, but there was considerable overlap in both age and renal variables. Our findings agree with the study by Williams and colleagues,11 in which pretreatment concentrations of urea nitrogen and creatinine correlated with development of post‐treatment azotemia. However, one of our most important clinical findings was that cats that developed azotemia after 131I treatment were much more likely to have “less‐than‐maximally concentrated” USG (<1.035) before 131I treatment than cats that remained nonazotemic (92% versus 29%, respectively). Our results are in contrast to what has been reported in some studies1, 6, 9 but are similar to others.3, 11 The reasons for the differences are not clear, but we followed these cats longer than many of the other studies (which we consider important, because post‐treatment azotemia might not develop until 6 months after treatment),4, 12 and we verified persistent (and generally progressive azotemia) together with USG <1.035 to help ensure that the cats were categorized correctly into azotemic and nonazotemic groups. In addition, most other studies failed to account for the cats that developed iatrogenic hypothyroidism, which could lower GFR and increase serum creatinine concentrations; such hypothyroid‐induced azotemia could be reversible with thyroid supplementation and might not represent true azotemic CKD.4, 12, 42
After 131I treatment, serum concentrations of SDMA, creatinine, and urea nitrogen all increased in both azotemic and nonazotemic cats, although serum concentrations of all 3 biomarkers were higher in the azotemic cats. Both groups of cats regained lost muscle mass after 131I treatment, which likely contributed to the increases in serum creatinine observed in these nonazotemic cats. However, the decrease in renal blood flow and GFR associated with cure of hyperthyroidism appears to be the main reason that all serum biomarker of renal function increased after 131I treatment.
One might question the long follow‐up time in this study, because 4–8 months (median time, 6 months) could be considered a long time between 131I and follow‐up, allowing new kidney disease to develop rather than simply “unmasking” of azotemia after treatment. We chose this follow‐up time because it is clear that even after high serum T4 concentrations normalize (by 1 month in most cats, but sometimes as long as 3 months), the serum creatinine concentrations can continue to increase over the next 3–6 months.1, 3, 4 This is the reason that short‐term methimazole trials (<3 months) may fail to predict pre‐azotemic CDK.12 Our median follow‐up time of 6 months provided a period long enough for azotemia to develop, if the cat was in the pre‐azotemic group. Additionally, this time allowed us to confirm the presence of azotemia (creatinine >2.1 mg/dL) by rechecking a month or so later.
In this study, 53 of these treated cats developed iatrogenic hypothyroidism, despite 131I doses that were much lower that given in most other studies—our median dose of 131I was only 1.9 mCi, approximately half that used by other investigators.43, 44, 45, 46 Most of these hypothyroid cats maintained a low‐normal serum T4 but had a high TSH concentration, a condition termed mild or subclinical hypothyroidism.32, 47, 48 Only 5 (1.9%) cats developed overt hypothyroidism (low T4 with high TSH concentration), resulting in a much lower prevalence of overt hypothyroidism than that reported after administration of higher 131I doses.43, 44, 45, 46 The prevalence of subclinical and overt hypothyroidism in the cats of this study is similar to those that we have previously reported in other cats treated with low‐dose 131I.28
Our study confirmed that cats with iatrogenic hypothyroidism are more likely to become azotemic than cats that remain euthyroid.11, 14 Additionally, the proportion of cats that develop azotemia after treatment for hyperthyroidism depends, at least in part, on the degree to which the hyperthyroidism is controlled (ie, how low the serum T4 falls).11, 14 Importantly, azotemia is more likely to develop in cats with overt hypothyroidism than in cats with subclinical hypothyroidism or in 131I‐treated cats that become euthyroid.11, 13, 14, 28 As in previous studies, these cats with overt hypothyroidism had a higher prevalence of azotemia (80%) than did the cats with milder, subclinical hypothyroidism (31%). Overall, these hypothyroid cats had a prevalence of azotemia that was 3‐fold higher than that of the 131I‐treated euthyroid cats (35.9% versus 11%)—essentially, half of all iatrogenic hypothyroid cats had “unmasked” azotemic CKD.
When all 131I‐treated cats were categorized as euthyroid or hypothyroid, the post‐treatment serum SDMA concentrations were higher in hypothyroid cats than euthyroid cats. This increase in serum SDMA concentration in the hypothyroid cats could be expected, as hypothyroidism in known to decrease GFR both in dogs42 and humans,49, 50 which can sometimes be normalized with thyroid hormone replacement alone.51, 52, 53 Direct GFR measurements have not been reported in hypothyroid cats—neither before nor after levothyroxine replacement—but those studies are needed. We recently reported, however, that levothyroxine can lower serum creatinine concentrations in 131I‐induced (iatrogenic) hypothyroid cats with moderate azotemia,14 but we know of no studies on the effect of levothyroxine on serum SDMA concentrations in hypothyroid cats—additional studies are again needed.
In cats that develop azotemia after treatment of hyperthyroidism, these findings emphasize the importance of identifying and treating iatrogenic hypothyroidism. Inasmuch as hypothyroidism might not develop for as long as 6–12 months after 131I treatment,1, 4, 11 it is important to monitor for hypothyroidism (serum T4 and TSH) during this period. If high serum TSH concentration together with low to low‐normal T4 concentration is documented, levothyroxine supplementation or adjustment of antithyroid medication should be considered, especially if azotemia has developed.
One limitation of this study was that we did not measure GFR directly. Because serum creatinine concentrations remain within the reference interval until over 75% of renal function has been lost,21 it is certainly possible that some of these clinically normal cats or 131I‐treated nonazotemic cats might have had subclinical, azotemic CKD that was missed. It is also possible that we misclassified a cat with azotemia (based on the finding of persistently high serum creatinine concentrations) that would have had a normal GFR on direct measurement. All of these deficiencies could have potentially confounded the comparison in serum SDMA concentrations among these healthy older cats and pre‐azotemic and nonazotemic hyperthyroid cats.
Our findings suggest that serum SDMA concentration and USG can help identify and predict, albeit imperfectly, hyperthyroid cats that are likely to develop azotemia after 131I treatment. Clinicians should evaluate these analytes before treatment and carefully monitor cats considered “high risk.” Furthermore, clinicians should carefully monitor cats for iatrogenic hypothyroidism, as these cats are much more likely to develop post‐treatment azotemia and high SDMA concentrations than cats made euthyroid.
Acknowledgments
Conflict of Interest Declaration
M. Peterson has received honoraria from IDEXX Laboratories.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Where Work was Performed: Animal Endocrine Clinic, New York, New York.
Presentation: Preliminary results of this study were presented at the 2017 ISFM World Feline Congress, Brighton, England.
Footnotes
AU5800 Clinical Chemistry System, Beckman Coulter, Brea, CA
Prusevich P, Patch D, Obare E, et al. Validation of a novel high throughput immunoassay for the quantitation of symmetric dimethylarginine (SDMA) [American Association for Clinical Chemistry 2015, abstract B‐048]. Clin Chem 2015;16:S135
Patch D, Obare E, Prusevich P, et al. High throughput immunoassay for kidney function biomarker symmetric dimethylarginine (SDMA) [American Association for Clinical Chemistry 2015, abstract B‐047]. Clin Chem 2015;16:S135
IDEXX Reference Laboratories, Westbrook, ME
GraphPad Prism, version 7.0; GraphPad Software, La Jolla, CA
MedCalc, version 17.6, MedCalc Software, bvnv, Ostend, Belgium
References
- 1. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec 2007;161:711–715. [DOI] [PubMed] [Google Scholar]
- 2. Graves TK, Olivier NB, Nachreiner RF, et al. Changes in renal function associated with treatment of hyperthyroidism in cats. Am J Vet Res 1994;55:1745–1749. [PubMed] [Google Scholar]
- 3. van Hoek I, Lefebvre HP, Peremans K, et al. Short‐ and long‐term follow‐up of glomerular and tubular renal markers of kidney function in hyperthyroid cats after treatment with radioiodine. Domest Anim Endocrinol 2009;36:45–56. [DOI] [PubMed] [Google Scholar]
- 4. Vaske HH, Schermerhorn T, Grauer GF. Effects of feline hyperthyroidism on kidney function: A review. J Feline Med Surg 2016;18:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peterson ME, Castellano CA, Rishniw M. Evaluation of body weight, body condition, and muscle condition in cats with hyperthyroidism. J Vet Intern Med 2016;30:1780–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becker TJ, Graves TK, Kruger JM, et al. Effects of methimazole on renal function in cats with hyperthyroidism. J Am Anim Hosp Assoc 2000;36:215–223. [DOI] [PubMed] [Google Scholar]
- 7. Slater MR, Geller S, Rogers K. Long‐term health and predictors of survival for hyperthyroid cats treated with iodine 131. J Vet Intern Med 2001;15:47–51. [DOI] [PubMed] [Google Scholar]
- 8. Adams WH, Daniel GB, Legendre AM, et al. Changes in renal function in cats following treatment of hyperthyroidism using 131‐I. Vet Radiol Ultrasound 1997;38:231–238. [DOI] [PubMed] [Google Scholar]
- 9. Riensche MR, Graves TK, Schaeffer DJ. An investigation of predictors of renal insufficiency following treatment of hyperthyroidism in cats. J Feline Med Surg 2008;10:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milner RJ, Channell CD, Levy JK, et al. Survival times for cats with hyperthyroidism treated with iodine 131, methimazole, or both: 167 cases (1996–2003). J Am Vet Med Assoc 2006;228:559–563. [DOI] [PubMed] [Google Scholar]
- 11. Williams TL, Peak KJ, Brodbelt D, et al. Survival and the development of azotemia after treatment of hyperthyroid cats. J Vet Intern Med 2010;24:863–869. [DOI] [PubMed] [Google Scholar]
- 12. Syme H. Are methimazole trials really necessary? In: Little SE, ed. August's Consultations in Feline Internal Medicine. St. Louis, MO: Elsevier; 2015:276–281. [Google Scholar]
- 13. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010;24:1086–1092. [DOI] [PubMed] [Google Scholar]
- 14. Peterson ME, Nichols R, Rishniw M. Serum thyroxine and thyroid‐stimulating hormone concentration in hyperthyroid cats that develop azotaemia after radioiodine therapy. J Small Anim Pract 2017;58:519–530. [DOI] [PubMed] [Google Scholar]
- 15. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014;28:1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Relford R, Robertson J, Clements C. Symmetric dimethylarginine: Improving the diagnosis and staging of chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract 2016;46:941–960. [DOI] [PubMed] [Google Scholar]
- 17. Tain YL, Hsu CN. Toxic dimethylarginines: Asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). Toxins (Basel) 2017;9:92. [Google Scholar]
- 18. Kielstein JT, Salpeter SR, Bode‐Boeger SM, et al. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function–a meta‐analysis. Nephrol Dial Transplant 2006;21:2446–2451. [DOI] [PubMed] [Google Scholar]
- 19. Braff J, Obare E, Yerramilli M, et al. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med 2014;28:1699–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jepson RE, Syme HM, Vallance C, et al. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l‐arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med 2008;22:317–324. [DOI] [PubMed] [Google Scholar]
- 21. Polzin DJ. Chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract 2011;41:15–30. [DOI] [PubMed] [Google Scholar]
- 22. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L‐carnitine, and medium‐chain triglycerides. Vet J 2014;202:588–596. [DOI] [PubMed] [Google Scholar]
- 23. Braun JP, Lefebvre HP, Watson AD. Creatinine in the dog: A review. Vet Clin Pathol 2003;32:162–179. [DOI] [PubMed] [Google Scholar]
- 24. Butani L, Polinsky MS, Kaiser BA, et al. Dietary protein intake significantly affects the serum creatinine concentration. Kidney Int 2002;61:1907. [DOI] [PubMed] [Google Scholar]
- 25. Relford R. Hyperthyroid cats: The IDEXX SDMA test is a more reliable indicator of kidney function than creatinine. Todays Vet Pract 2017;7:112–113. [Google Scholar]
- 26. Hermenegildo C, Medina P, Peiro M, et al. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in hyperthyroid patients. J Clin Endocrinol Metab 2002;87:5636–5640. [DOI] [PubMed] [Google Scholar]
- 27. Arikan E, Karadag CH, Guldiken S. Asymmetric dimethylarginine levels in thyroid diseases. J Endocrinol Invest 2007;30:186–191. [DOI] [PubMed] [Google Scholar]
- 28. Lucy JM, Peterson ME, Randolph JF, et al. Efficacy of low‐dose (2 millicurie) versus standard‐dose (4 millicurie) radioiodine treatment for cats with mild‐to‐moderate hyperthyroidism. J Vet Intern Med 2017;31:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peterson ME, Guterl JN, Nichols R, et al. Evaluation of serum thyroid‐stimulating hormone concentration as a diagnostic test for hyperthyroidism in cats. J Vet Intern Med 2015;29:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterson ME, Broome MR. Thyroid scintigraphy findings in 2,096 cats with hyperthyroidism. Vet Radiol Ultrasound 2015;56:84–95. [DOI] [PubMed] [Google Scholar]
- 31. Peterson ME, Guterl JN, Rishniw M, et al. Evaluation of quantitative thyroid scintigraphy for diagnosis and staging of disease severity in cats with hyperthyroidism: Comparison of the percent thyroidal uptake of pertechnetate to the thyroid‐to‐salivary ratio and thyroid‐to‐background ratios. Vet Radiol Ultrasound 2016;57:427–440. [DOI] [PubMed] [Google Scholar]
- 32. Peterson ME. Diagnosis and management of iatrogenic hypothyroidism In: Little SE, ed. August's Consultations in Feline Internal Medicine. St. Louis, MO: Elsevier; 2016:260–269. [Google Scholar]
- 33. Jacobs RM, Lumsden JH, Taylor JA, et al. Effects of interferents on the kinetic Jaffe reaction and an enzymatic colorimetric test for serum creatinine concentration determination in cats, cows, dogs and horses. Can J Vet Res 1991;55:150–154. [PMC free article] [PubMed] [Google Scholar]
- 34. D'Agostino RB. Tests for normal distribution In: D'Agostino RB, Stephens MA, eds. Goodness‐of‐Fit Techniques. New York, NY: Macel Dekker; 1986:367–420. [Google Scholar]
- 35. Corder GW, Foreman DI. Nonparametric Statistics for Non‐Statisticians: A Step‐by‐Step Approach. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 36. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012;41:441–453. [DOI] [PubMed] [Google Scholar]
- 37. Dunn OJ. Multiple contrasts using rank sums. Technometrics 1964;5:241–252. [Google Scholar]
- 38. Akobeng AK. Understanding diagnostic tests 1: Sensitivity, specificity and predictive values. Acta Paediatr 2007;96:338–341. [DOI] [PubMed] [Google Scholar]
- 39. Gardner IA, Greiner M. Receiver‐operating characteristic curves and likelihood ratios: Improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet Clin Pathol 2006;35:8–17. [DOI] [PubMed] [Google Scholar]
- 40. Nick TG, Campbell KM. Logistic regression. Methods Mol Biol 2007;404:273–301. [DOI] [PubMed] [Google Scholar]
- 41. Ittermann T, Bahls M, Atzler D, et al. L‐arginine derivatives are associated with the hyperthyroid state in the general population. Thyroid 2016;26:212–218. [DOI] [PubMed] [Google Scholar]
- 42. Panciera DL, Lefebvre HP. Effect of experimental hypothyroidism on glomerular filtration rate and plasma creatinine concentration in dogs. J Vet Intern Med 2009;23:1045–1050. [DOI] [PubMed] [Google Scholar]
- 43. Chun R, Garrett LD, Sargeant J, et al. Predictors of response to radioiodine therapy in hyperthyroid cats. Vet Radiol Ultrasound 2002;43:587–591. [DOI] [PubMed] [Google Scholar]
- 44. Craig A. A prospective study of 66 cases of feline hyperthyroidism treated with a fixed dose of intravenous 131‐I. Aust Vet Pract 1993;23:2–6. [Google Scholar]
- 45. Klausner JS. Results of radioactive iodine therapy in 23 cats with hyperthyroidism. Minn J Vet Med 1987;27:28–31. [Google Scholar]
- 46. Nykamp SG, Dykes NL, Zarfoss MK, et al. Association of the risk of development of hypothyroidism after iodine 131 treatment with the pretreatment pattern of sodium pertechnetate Tc 99 m uptake in the thyroid gland in cats with hyperthyroidism: 165 cases (1990–2002). J Am Vet Med Assoc 2005;226:1671–1675. [DOI] [PubMed] [Google Scholar]
- 47. Cooper DS. Clinical practice. Subclinical hypothyroidism. N Engl J Med 2001;345:260–265. [DOI] [PubMed] [Google Scholar]
- 48. Fatourechi V. Subclinical hypothyroidism: An update for primary care physicians. Mayo Clin Proc 2009;84:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab 2012;16:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dousdampanis P, Trigka K, Vagenakis GA, et al. The thyroid and the kidney: A complex interplay in health and disease. Int J Artif Organs 2014;37:1–12. [DOI] [PubMed] [Google Scholar]
- 51. Gommeren K, van Hoek I, Lefebvre HP, et al. Effect of thyroxine supplementation on glomerular filtration rate in hypothyroid dogs. J Vet Intern Med 2009;23:844–849. [DOI] [PubMed] [Google Scholar]
- 52. Hataya Y, Igarashi S, Yamashita T, et al. Thyroid hormone replacement therapy for primary hypothyroidism leads to significant improvement of renal function in chronic kidney disease patients. Clin Exp Nephrol 2013;17:525–531. [DOI] [PubMed] [Google Scholar]
- 53. Mansourian AR. A literature review on the adverse effects of hypothyroidism on kidney function. Pak J Biol Sci 2012;15:709–719. [DOI] [PubMed] [Google Scholar]


