Abstract
Background
Symmetric dimethylarginine (SDMA) has been increasingly used as a marker of early chronic kidney disease (CKD) in cats, but little is known about the influence of comorbidities on SDMA in this species.
Hypothesis
Hypertrophic cardiomyopathy (HCM) and diabetes mellitus (DM), independently of CKD, are associated with changes in serum SDMA.
Animals
Ninety‐four cats (17 with CKD, 40 with HCM, 17 with DM, and 20 healthy controls).
Methods
Case‐control study. Clinical examination, echocardiography, ECG, blood pressure, CBC, biochemistry, thyroxine, and SDMA measurement were performed. Urinalysis was performed in controls and cats with CKD and DM. Analysis of variance was used to compare overall differences in the log‐transformed SDMA data among groups. A random forest algorithm was applied to explore which clinical and other factors influenced serum SDMA.
Results
Median (range) serum SDMA for the renal group (positive control) was 19 (10–93) μg/dL, whereas for the control group (negative control), it was 10 (5–15) μg/dL. For the cardiac and diabetic groups, serum SDMA was 9 (4–24) μg/dL and 7 (3–11) μg/dL, respectively. The renal group had significantly higher SDMA concentrations and the diabetic group significantly lower SDMA concentrations compared to all other groups.
Conclusions and Clinical Importance
Serum SDMA concentrations in cats with HCM were not significantly different from those of healthy control cats. Cats with DM, however, had significantly lower SDMA concentrations than controls, a finding that needs further investigation and should be kept in mind when evaluating renal function of cats with this endocrinopathy.
Keywords: Biomarker, Cardiology, Companion animals, Endocrinology
Abbreviations
- ANOVA
analysis of variance
- CKD
chronic kidney disease
- DM
diabetes mellitus
- GFR
glomerular filtration rate
- HCM
hypertrophic cardiomyopathy
- IRIS
International Renal Interest Society
- IVSd
interventricular septal thickness at end‐diastole
- LVFWd
left ventricular free wall thickness at end‐diastole
- NO
nitric oxide
- RMSE
root‐mean‐squared error
- SAM
systolic anterior motion
- SDMA
symmetric dimethylarginine
Chronic kidney disease (CKD) is the most common metabolic disease in cats.1 Its prevalence increases with age, reaching >80% in cats older than 15 years.2 Although the diagnosis is often straight forward once cats reach azotemic stages of disease, earlier diagnosis is desirable because recent studies suggest a possible benefit of initiating renoprotective nutritional treatment before this stage.3, 4
Symmetric dimethylarginine (SDMA) is a by‐product of normal intracellular protein metabolism, released after symmetric methylation of arginine residues and subsequent protein hydrolysis.5, 6 The molecule is almost solely cleared by glomerular filtration, where it is freely filtered because of its small molecular size and positive charge.5 In cats with CKD, SDMA has an inverse linear relationship with glomerular filtration rate (GFR)7 and increases when GFR decreases by approximately 25%, with a mean time of 17 months before an increase in serum creatinine concentration.8 Consequently, SDMA has become a renal marker of considerable interest in recent years.
Although SDMA reflects kidney function, it has not been thought to affect it or to directly contribute to progression of renal disease.9 A recent study, however, suggested that SDMA might not be an inert molecule with respect to renal pathology.10 Additionally, SDMA appears to play an important role in vascular homeostasis, and thus the effects of and on SDMA of nonrenal disease still are being clarified. Older cats with CKD frequently are afflicted with comorbidities, 2 of the most common being hypertrophic cardiomyopathy (HCM) and diabetes mellitus (DM). Eliminating an influence of such comorbidities on SDMA in cats is important to confirm its reliability as a renal marker.
One study of HCM in humans reported increased serum SDMA concentrations in patients with this disease.11 The cause has not been determined with certainty, but the effect may be endothelial dysfunction of which SDMA is believed to be a mediator.6 The vascular endothelium releases vasoactive substances such as nitric oxide (NO) which help maintain vascular integrity and regulate its homeostasis.12, 13 Nitric oxide influences vascular tone, blood pressure, and regional blood flow while also having anti‐inflammatory, antithrombotic, and antiatherosclerotic effects.6 SDMA is believed to decrease NO synthesis by competing with its substrate, arginine, for cellular uptake.14, 15 Additionally, endothelial dysfunction also may be mediated through proinflammatory effects of SDMA on the vascular wall.10, 16 Because abnormal coronary vasculature is believed to be an important factor in the pathophysiology of HCM in cats, as in humans,11, 17 evaluating SDMA in cats with HCM is of interest.
In humans with DM, interpretation of SDMA is complicated because of the frequent development of diabetic nephropathy.18, 19, 20 In cats, however, renal disease does not appear to occur more commonly in diabetic individuals than in age‐matched controls.21 Therefore, a possible influence of DM on SDMA might, in fact, be more apparent in cats than in humans.
The aim of our study was to establish whether HCM and DM, independently of CKD, are associated with changes in serum SDMA in cats.
Materials and Methods
Cats were categorized according to disease as a renal group (cats with azotemic renal disease defined as serum creatinine concentration >140 μmol/L and urine specific gravity <1.035), a cardiac group (cats with HCM defined as maximal end‐diastolic thickness of the interventricular septum or left ventricular caudal free wall >5.5 mm according to a conventional echocardiographic protocol),22 a diabetic group (cats with DM defined as hyperglycemia and glucosuria in the presence of corresponding clinical signs), and a healthy control group (cats deemed healthy based on clinical examination, echocardiography, ECG, and clinical pathology [CBC, biochemistry, urinalysis, and serum thyroxine results]). All 4 groups of cats were included retrospectively from previous prospective studies performed by the authors. The renal group was included as a positive control and the healthy control group as a negative control for the 2 groups of cats to be investigated (cardiac and diabetic groups). Each of the prospective studies had been approved by the ethical committee of the Department of Veterinary Clinical Sciences, University of Copenhagen, Denmark, and informed owner consent for participation had been obtained.
All cats had been included through the internal medicine and cardiology services at the University Hospital for Companion Animals, University of Copenhagen, Denmark. For all cats, a clinical examination had been carried out, and echocardiography, ECG, CBC, biochemistry, and thyroxine measurement had been performed. Blood pressure measurements had been performed for the majority of cats. Urinalysis had been performed for all cats in the renal, diabetic, and control groups.
Cats were excluded from the study if they were <1 year of age. For each of the 3 disease groups, cats with evidence of comorbidities were excluded. Cats with HCM were excluded if abnormalities on CBC, biochemistry, serum thyroxine concentration, or blood pressure were identified. Because urinalyses and abdominal ultrasound examinations were not available for this group of cats, the risk of inclusion of cats with stage 1 CKD according to guidelines of the International Renal Interest Society (IRIS) could not be eliminated, but all cats with serum creatinine concentration >140 μmol/L were excluded. Cats with DM were excluded if abnormalities on CBC, biochemistry (other than those associated with DM), serum thyroxine concentration, or echocardiography were identified. Cats with renal disease were excluded if abnormalities on CBC, biochemistry (other than those associated with CKD), serum thyroxine concentration, or echocardiography were identified.
Serum for measurement of SDMA was obtained from the departmental biobank. Serum had been collected into 4 mL gel separator tubes, allowed to clot for 15 minutes at room temperature, centrifuged at 2,500×g for 5 minutes, separated, and stored in cryovials at −80°C within 6 hours of blood collection. Samples had been stored for a maximum of 6 years before thawing and shipping to IDEXX laboratories1 for SDMA analysis.
Statistical Analysis
Data were analyzed by commercial statistical software.2 Normality of data was assessed visually by histograms and qq‐plots. If data were not found to be normally distributed, they were log‐transformed and reassessed. Analysis of variance (ANOVA) with age as a cofactor was used to compare overall differences in the log‐transformed SDMA data among the 4 groups; cardiac, diabetic, renal, and control, respectively. Posthoc analysis of the 2‐way ANOVA then was performed by Tukey Honest Significant Difference. Spearman correlation was used to analyze the degree of correlation between SDMA and the variables age, body weight, blood pressure (systolic, diastolic, and mean arterial pressure), hematocrit, serum creatinine concentration, blood urea nitrogen concentration, and thyroxine concentration. P‐values were adjusted by false discovery rate q‐values and were considered significant when <0.05. To explore which factors most significantly influenced SDMA, a random forest algorithm was trained by 10‐fold cross‐validation with 80% of the data, and subsequently tested on the remaining 20% of the data.23 Variables included in training the regression model included those for which correlation analysis was performed as well as group (cardiac, diabetic, renal, control), breed, sex, neutering, and, for the cardiac group, the presence or absence of congestive heart failure or systolic anterior motion of the anterior mitral leaflet (SAM). Splitter importance was computed on the basis of minimizing the root‐mean‐squared error (RMSE), and the 10 most important features from the random forest model were extracted.
Results
The study population was drawn from a total of 121 cats. Twenty‐seven cats were excluded based on the above criteria, leaving a final study population of 94 cats distributed as 17 cats in the renal group (16 cats with CKD, 1 cat with CKD and obstructive ureterolithiasis), 40 cats in the cardiac group (all with HCM, 16 of which had SAM and 5 of which were in congestive heart failure based on clinical and echocardiographic findings), 17 cats in the diabetic group, and 20 healthy control cats. Demographic data for each group are presented in Table 1. Blood pressure measurements were available for 15 cats in the renal group, all cats in the cardiac group, 10 cats in the diabetic group, and 10 healthy controls.
Table 1.
Demographic data of included cats
| Group | n | Breeds | Sex | Age (years) | Body Weight (kg) |
|---|---|---|---|---|---|
| Renal | 17 | 11 DSH, 2 NFC, 2 DLH, 1 RB, 1 MB | 7 FN, 10 MN | 11 (4–17) | 4.8 (2.4–7.0) (n = 15) |
| Cardiac | 40 | 17 MC, 15 BSH, 4 NFC, 2 ESH, 2 DSH | 4 FE, 9 FN, 4 ME, 23 MN | 6.5 (1–15) | 6.0 (3.4–8.6) (n = 39) |
| Diabetic | 17 | 12 DSH, 2 NFC, 3 Ocicats | 5 FN, 1 ME, 11 MN | 9 (6–16) | 5.8 (3.2–8.9) (n = 17) |
| Control | 20 | 11 DSH, 4 BSH, 2 NFC, 2 Somalis, 1 Burmese | 3 FE, 11 FN, 6 MN | 7 (3–15) | 5.0 (3.4–6.8) (n = 19) |
BSH, British shorthair; DSH, Domestic shorthair; DLH, Domestic longhair; ESH, Exotic shorthair; MB, Mixed breed; MC, Maine coon; NFC, Norwegian forest cat; RB, Russian blue; FE, Female entire; FN, Female neutered; ME, Male entire; MN, Male neutered.
Median (range) serum SDMA for the renal group (positive control) was 19 (10–93) μg/dL, whereas for the control group (negative control), it was 10 (5–15) μg/dL. For the cardiac and diabetic groups, serum SDMA was 9 (4–24) μg/dL and 7 (3–11) μg/dL, respectively.
The renal group had significantly higher serum SDMA and creatinine concentrations compared to all other groups, whereas the diabetic group had significantly lower serum SDMA and creatinine concentrations compared to all other groups. No significant difference was found between the cardiac group and the control group for either variable (Figs 1 and 2). No significant influence on SDMA concentration was found for either age (P = 0.17) or interaction between age and group (P = 0.42). However, a significant influence on serum creatinine concentration was found for the interaction between age and group (P = 0.006), but not for age alone (P = 0.46).
Figure 1.
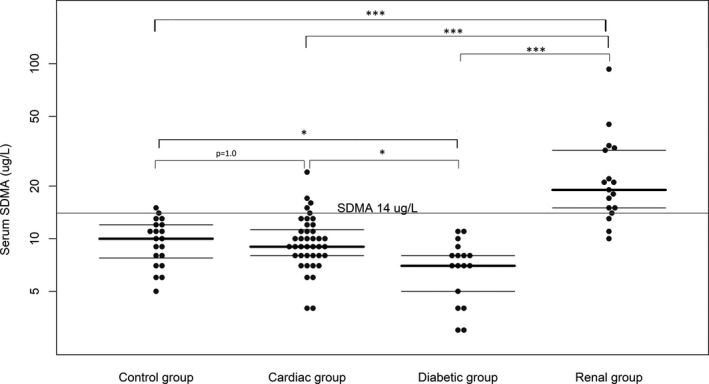
Serum SDMA concentrations of the control group, diabetic group, cardiac group, and renal group. Median and interquartile ranges are shown as horizontal lines. Statistical significance between groups is symbolized as P < 0.05 = *, P < 0.01 = **, or P < 0.001 = ***.
Figure 2.
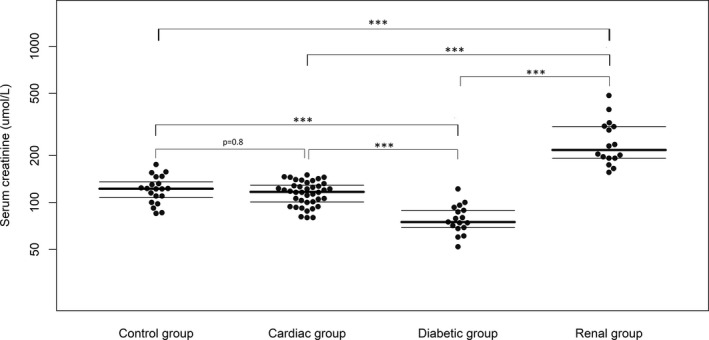
Serum creatinine concentrations of the control group, diabetic group, cardiac group, and renal group. Median and interquartile ranges are shown as horizontal lines. Statistical significance between groups is symbolized as P < 0.05 = *, P < 0.01 = **, or P < 0.001 = ***.
Correlation analyses identified a significant correlation only between serum SDMA and creatinine concentrations (R 2 = 0.53; P < 0.001).
The final random forest model applied to the data was selected after 10‐fold cross‐validation by generation of 500 trees. Model results were R 2 = 0.39 and RMSE = 1.96 when evaluated on the randomly selected 20% of the data not used for model training (the test data). Predictors with the 10 highest variable importance scores are shown in Figure 3.
Figure 3.
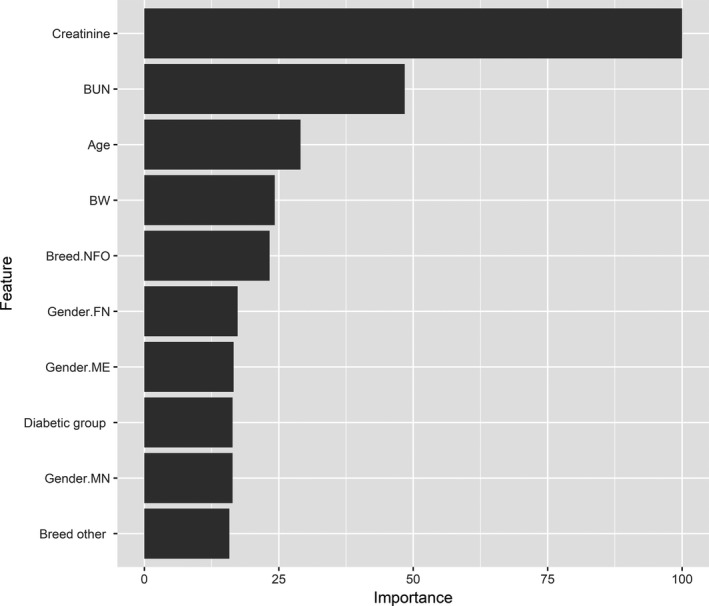
Predictors of SDMA in a population of 94 cats (control group, diabetic group, cardiac group, and renal group) were determined through a random forest algorithm including the variables age, body weight, blood pressure (systolic, diastolic, and mean arterial pressure), hematocrit, creatinine, blood urea nitrogen, thyroxine, group, breed, sex, neutering, and, for the cardiac group, the presence or absence of systolic anterior motion of the anterior mitral leaflet (SAM) and congestive heart failure. Predictors with the top 10 highest variable importance scores are presented. BUN, Blood urea nitrogen; BW, Body weight; NFO, Norwegian forest cat; FN, Female neutered; ME, Male entire; MN, Male neutered.
Discussion
Our study investigated the possible influence of HCM and DM on serum SDMA in cats.
Unlike what has been reported in humans,11 cats with HCM in our study did not have serum SDMA concentrations significantly different from those of healthy controls. The cause of increased serum SDMA concentrations in humans with HCM has not been determined, but it has been speculated to be related to shear stress which increases the activity of arginine methyltransferase,24 the enzyme responsible for production of SDMA and its counterpart, asymmetric dimethylarginine. The obstructive form of HCM, which is associated with higher shear stress, is associated with the highest SDMA concentrations in humans.11 Shear stress in cats with HCM is most evident with the frequent occurrence of SAM,25 which was present in 16 of 40 cats in our study. Interestingly, SAM was not among the variables identified by the random forest model as important predictors of serum SDMA in our study.
Although most of the included cats had occult heart disease, 5 cats had clinical and echocardiographic evidence of congestive heart failure at the time of presentation. Previous studies in humans and pigs have suggested an association between serum SDMA and severity of cardiac disease26, 27 In our study, however, there was no indication of increasing serum SDMA concentrations in cats with heart failure. In fact, this small group of cats had serum SDMA concentrations in the lower half of the cardiac group range, and HCM as a whole did not appear to influence serum SDMA. Nevertheless, a study including a larger number of cats with more severe stages of heart disease and no concurrent renal disease would be required to confirm this finding.
Keeping in mind the effect of SDMA on endothelial function described in humans, it is perhaps not surprising that the biomarker has been associated with the severity of coronary atherosclerosis.26, 28 In humans with HCM, evidence of endothelial dysfunction has been detected along with and possibly caused by a simultaneous increase in serum SDMA.11 Although abnormal coronary vasculature also is believed to be an important part of the pathophysiology in HCM in cats,11, 17 atherosclerosis is uncommon in this species.29 Considering our results, the link between serum SDMA and endothelial dysfunction might not be as evident in cats.
Another important finding of our study was the significantly lower serum SDMA concentrations in cats with DM compared to controls. It was mirrored by concurrently lower serum creatinine concentrations in the same group of cats. Previous studies have not found cats with DM to have lower serum creatinine concentrations compared to healthy cats, but these studies included DM cats with concomitant CKD which may have obscured the results.21, 30 Serum creatinine concentration is known to decrease with muscle wasting in older animals, which may offset the increase expected to occur as GFR decreases. However, SDMA is not affected by muscle wasting, and an effect of age on SDMA previously detected in older cats was found to be related to older cats having lower GFR.31 Accordingly, the decreased serum SDMA concentrations of the diabetic group are not believed to be related to muscle wasting, but considered related to the disease itself, a finding that was supported by the random forest analysis in which belonging to the diabetic group was among the most important predictors of SDMA. Nevertheless, weight also was a predictor in the analysis, but cats with DM had comparable weight to those of the control group (Fig 3, Table 1). Optimally, a specific measure of muscle mass rather than weight would have been beneficial for evaluation of these cats, and, in the absence of such information, the cause of the lower serum creatinine concentration in this group cannot be conclusively determined.
In humans, unlike in cats, diabetic nephropathy is the leading cause of renal disease.18 Diabetic patients furthermore have increased risk of clinical cardiovascular disease.32 Higher concentrations of serum SDMA, therefore, would be expected to be more frequent in humans with DM. However, results are conflicting. One study19 found SDMA to be a reliable marker of GFR in children with type 1 DM, whereas another study20 found lower serum SDMA concentrations in people with poorly controlled type 2 DM than in healthy controls. In accordance with the latter study, it has been shown that humans with evidence of insulin resistance have lower serum SDMA concentrations.13, 33 Possible mechanisms include increased cellular uptake or enhanced elimination in response to hyperfiltration.33 Additionally, it has been suggested that hepatic metabolism of SDMA could be upregulated in patients with insulin resistance.33, 34 Insulin resistance is known to be an important factor in development of DM in cats,35 an interesting fact, because results of our study mirror studies of humans with type 2 DM. Pathophysiologically, it is likely that hyperfiltration and osmotic diuresis are the most important factors leading to decreased serum SDMA concentrations in DM, especially in patients with poorly controlled DM. Of the 17 cats with DM included in our study, 6 were newly diagnosed, and 4 were still undergoing adjustment of their insulin treatment, which would make hyperfiltration particularly likely in the diabetic group. Furthermore, 1 cat was excluded from the study because of concomitant CKD and DM. Interestingly, this cat had a normal serum SDMA concentration despite CKD, which possibly could be related to concurrent DM. Accordingly, although these findings should be confirmed by future prospective studies, SDMA might be less valuable as a test of early CKD in cats with DM.
Interestingly, although the renal group was included as the positive control of our study, 5 cats with renal disease did, in fact, have serum SDMA concentrations within the reference range. The cause of this finding is unknown, but serves as a reminder that SDMA does not have perfect sensitivity, and always should be interpreted in light of other clinical information. Similarly, 1 healthy control cat did have a mildly increased serum SDMA concentration. We chose to not exclude this cat from the study to avoid biasing the data by defining groups by the variable on which they were compared. Repeated data analysis without this cat, however, did not alter the conclusions. Early renal disease in this cat is a possibility, and optimally should have been investigated by GFR measurement.
The main limitation of our study is its retrospective design. However, both cases and controls were included from previous prospective studies carried out by the authors, who had been personally responsible for the systematic data collection from each animal. The individual disease groups were rather small, especially when evaluating the subset of cats with congestive heart failure, and larger studies are required to confirm our findings. A specific limitation was lack of radiographic confirmation of congestive heart failure for this small group. However, all cats had severely enlarged left atria and corresponding clinical signs, and, for 4 of the 5 cats, confirmation of failure was achieved by echocardiographic detection of pleural fluid, pericardial fluid, or both. Serum sample storage time is also a possible limitation. Samples had been stored at −80°C for a maximum of 6 years, but, although long‐term stability studies on SDMA still are needed, a recent study in dogs showed that SDMA was unaffected by multiple freeze‐thaw cycles and stable after 2 weeks at room temperature.36 Hence, SDMA is considered a biomarker of high stability. Finally, an important limitation is the lack of available urinalysis data for the cardiac group. Although included cats were nonazotemic, some may have had IRIS stage 1 disease. One cat, moreover, was receiving furosemide treatment at presentation which also could have influenced its serum SMDA concentration. However, because the cardiac group had serum SDMA concentrations indistinguishable from those of the control group, these possible confounders are considered of limited importance.
In conclusion, we did not find an influence of HCM on serum SDMA concentration in cats. Therefore, SDMA may be a reliable marker of GFR in cats with HCM. Studies evaluating a larger number of cats with severe stages of HCM are required, however, to confirm these findings. Cats with DM had significantly lower serum SDMA concentrations than did healthy controls, a finding that needs further investigation. The possible effect of hyperfiltration should be kept in mind when evaluating renal function in cats with DM.
Acknowledgments
Grant support: All SDMA analyses were performed free of charge by IDEXX Laboratories.1
Conflict of Interest Declaration
IDEXX Laboratories performed the SDMA analyses for the study free of charge. IDEXX was not otherwise involved in the study and were only aware of the results that were presented at the ECVIM‐CA congress in Gothenburg 2016.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The study was performed at the Department of Veterinary Clinical Sciences, University of Copenhagen, Denmark.
Part of the study was presented as an oral abstract at the ECVIM‐CA Congress 2016: I.N. Kieler, L.R. Jessen, R. Langhorn, J. Koch, L.B. Christiansen. Feline hypertrophic cardiomyopathy does not alter serum levels of symmetric dimethylarginine. J Vet Intern Med 2017;31:207 (abstract).
Footnotes
IDEXX Laboratories. Vet Med Labor GmbH, Mörikestrasse 28/3, D‐71636 Ludwigsburg, Germany
“R” version 3.4.0. for Microsoft Windows. R Development Core Team (2008). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3‐900051‐07‐0, URL http://www.R-project.org
References
- 1. Brown CA, Elliott J, Schmiedt CW, Brown SA. Chronic kidney disease in aged cats. Vet Pathol 2016;53:309–326. [DOI] [PubMed] [Google Scholar]
- 2. Marino CL, Lascelles BDX, Vaden SL, et al. The prevalence and classification of chronic kidney disease in cats randomly selected within four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2015;16:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall JA, MacLeay J, Yerramilli M, et al. Positive impact of nutritional interventions on serum symmetric dimethylarginine and creatinine concentrations in client‐owned geriatric dogs. PLoS One 2016;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freeman LM, Lachaud MP, Matthews S, et al. Evaluation of weight loss over time in cats with chronic kidney disease. J Vet Intern Med 2016;30:1661–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Relford R, Robertson J, Clements C. Symmetric dimethylarginine: Improving the diagnosis and staging of chronic kidney disease in small animals. Vet Clin North Am ‐ Small Anim Pract 2016;46:941–960. [DOI] [PubMed] [Google Scholar]
- 6. Mangoni AA. The emerging role of symmetric dimethylarginine in vascular disease. Adv Clin Chem 2009;48:73–94. [DOI] [PubMed] [Google Scholar]
- 7. Braff J, Obare E, Yerramilli M, et al. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med 2014;28:1699–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014;28:1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Veldink H, Faulhaber‐Walter R, Park J‐K, et al. Effects of chronic SDMA infusion on glomerular filtration rate, blood pressure, myocardial function and renal histology in C57BL6/J mice. Nephrol Dial Transplant 2013;28:1434–1439. [DOI] [PubMed] [Google Scholar]
- 10. Schepers E, Barreto DV, Liabeuf S, et al. Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clin J Am Soc Nephrol 2011;6:2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dimitrow PP, Undas A, Bober M, et al. Plasma biomarkers of endothelial dysfunction in patients with hypertrophic cardiomyopathy. Pharmacol Rep 2007;59:715–720. [PubMed] [Google Scholar]
- 12. Jepson RE, Syme HM, Vallance C, Elliott J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, L‐Arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med 2008;22:317–324. [DOI] [PubMed] [Google Scholar]
- 13. Dimitroulas T, Hodson J, Sandoo A, et al. Symmetric dimethylarginine (SDMA) serum levels in rheumatoid arthritis: Correlations with insulin resistance and disease activity scores. Amino Acids 2015;47:1995–2004. [DOI] [PubMed] [Google Scholar]
- 14. Closs EI, Basha FZ, Habermeier A, Förstermann U. Interference of L‐arginine analogues with L‐arginine transport mediated by the y+ carrier hCAT‐2B. Nitric Oxide Biol Chem 1997;1:65–73. [DOI] [PubMed] [Google Scholar]
- 15. Bogle RG, MacAllister RJ, Whitley GS, Vallance P. Induction of NG‐monomethyl‐L‐arginine uptake: A mechanism for differential inhibition of NO synthases? Am J Physiol 1995;269:C750–C756. [DOI] [PubMed] [Google Scholar]
- 16. Schepers E, Glorieux G, Dhondt A, et al. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store‐operated calcium influx in monocytes. Nephrol Dial Transplant 2009;24:1429–1435. [DOI] [PubMed] [Google Scholar]
- 17. Liu SK, Roberts WC, Maron BJ. Comparison of morphologic findings in spontaneously occurring hypertrophic cardiomyopathy in humans, cats and dogs. Am J Cardiol 1993;72:944–951. [DOI] [PubMed] [Google Scholar]
- 18. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: A report from an ADA consensus conference. Diabetes Care 2014;37:2864–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marcovecchio ML, Dalton RN, Turner C, et al. Symmetric dimethylarginine, an endogenous marker of glomerular filtration rate, and the risk for microalbuminuria in young people with type 1 diabetes. Arch Dis Child 2010;95:119–124. [DOI] [PubMed] [Google Scholar]
- 20. Can A, Bekpinar S, Gurdol F, et al. Dimethylarginines in patients with type 2 diabetes mellitus: Relation with the glycaemic control. Diabetes Res Clin Pract 2011;94:e61–e64. [DOI] [PubMed] [Google Scholar]
- 21. Zini E, Benali S, Coppola L, et al. Renal morphology in cats with diabetes mellitus. Vet Pathol 2014;51:1143–1150. [DOI] [PubMed] [Google Scholar]
- 22. Langhorn R, Tarnow I, Willesen JL, et al. Cardiac Troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014;28:1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breiman L. Random forests. Mach Learn 2001;45:5–32. [Google Scholar]
- 24. Osanai T, Saitoh M, Sasaki S, et al. Effect of shear stress on asymmetric dimethylarginine release from vascular endothelial cells. Hypertension 2003;42:985–990. [DOI] [PubMed] [Google Scholar]
- 25. Schober K, Todd A. Echocardiographic assessment of left ventricular geometry and the mitral valve apparatus in cats with hypertrophic cardiomyopathy. J Vet Cardiol 2010;12:1–16. [DOI] [PubMed] [Google Scholar]
- 26. Kiechl S, Lee T, Santer P, et al. Asymmetric and symmetric dimethylarginines are of similar predictive value for cardiovascular risk in the general population. Atherosclerosis 2009;205:261–265. [DOI] [PubMed] [Google Scholar]
- 27. Cirera S, Moesgaard SG, Zois NE, et al. Plasma proANP and SDMA and microRNAs are associated with chronic mitral regurgitation in a pig model. Endocr Connect 2013;2:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bode‐Boger SM. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol 2006;17:1128–1134. [DOI] [PubMed] [Google Scholar]
- 29. Kidd L, Stepien RL, Amrheiw DP. Clinical findings and coronary artery disease in dogs and cats with acute and subacute myocardial necrosis: 28 cases. J Am Anim Hosp Assoc 2015;36:199–208. [DOI] [PubMed] [Google Scholar]
- 30. Paepe D, Ghys LF, Smets P, et al. Routine kidney variables, glomerular filtration rate and urinary cystatin C in cats with diabetes mellitus, cats with chronic kidney disease and healthy cats. J Feline Med Surg 2015;17:880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L‐carnitine, and medium‐chain triglycerides. Vet J 2014;202:588–596. [DOI] [PubMed] [Google Scholar]
- 32. Pasterkamp G. Methods of accelerated atherosclerosis in diabetic patients. Heart 2013;99:743–749. [DOI] [PubMed] [Google Scholar]
- 33. Zsuga J, Török J, Magyar MT, et al. Dimethylarginines at the crossroad of insulin resistance and atherosclerosis. Metabolism 2007;56:394–399. [DOI] [PubMed] [Google Scholar]
- 34. Siroen MPC, Van Der Sijp JRM, Teerlink T, et al. The human liver clears both asymmetric and symmetric dimethylarginine. Hepatology 2005;41:559–565. [DOI] [PubMed] [Google Scholar]
- 35. Nelson RW, Reusch CE. Animal models of disease: Classification and etiology of diabetes in dogs and cats. J Endocrinol 2014;222:T1–T9. [DOI] [PubMed] [Google Scholar]
- 36. Nabity MB, Lees GE, Boggess MM, et al. Symmetric dimethylarginine assay validation, stability, and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med 2015;29:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]


