Abstract
Key message
This study compares five models of GWAS, to show the added value of non-additive modeling of allelic effects to identify genomic regions controlling flowering time of sunflower hybrids.
Abstract
Genome-wide association studies are a powerful and widely used tool to decipher the genetic control of complex traits. One of the main challenges for hybrid crops, such as maize or sunflower, is to model the hybrid vigor in the linear mixed models, considering the relatedness between individuals. Here, we compared two additive and three non-additive association models for their ability to identify genomic regions associated with flowering time in sunflower hybrids. A panel of 452 sunflower hybrids, corresponding to incomplete crossing between 36 male lines and 36 female lines, was phenotyped in five environments and genotyped for 2,204,423 SNPs. Intra-locus effects were estimated in multi-locus models to detect genomic regions associated with flowering time using the different models. Thirteen quantitative trait loci were identified in total, two with both model categories and one with only non-additive models. A quantitative trait loci on LG09, detected by both the additive and non-additive models, is located near a GAI homolog and is presented in detail. Overall, this study shows the added value of non-additive modeling of allelic effects for identifying genomic regions that control traits of interest and that could participate in the heterosis observed in hybrids.
Electronic supplementary material
The online version of this article (doi:10.1007/s00122-017-3003-4) contains supplementary material, which is available to authorized users.
Keywords: Genome-wide association study, Sunflower, Multi-locus, Non-additive effect
Introduction
Currently, several tools are available to geneticists and breeders to identify the genetic control of traits of interest and to improve the performance of animals and plants. A powerful tool for mapping the genes controlling complex traits, association genetics essentially evaluates statistical correlations between the alleles at a given locus and the observed phenotype (Ersoz et al. 2007). Genome-wide association studies (GWAS) have been widely used in the genetics of humans, animals, and plants (Yu et al. 2006; Kang et al. 2008; Zhang et al. 2010; Zhou et al. 2012; Wang et al. 2016). The method was first applied to human genetics (Corder et al. 1993), and the first association study on agronomic data was conducted in 2001 (Thornsberry et al. 2001) in maize with regard to flowering time.
Flowering time (FT) is a key trait in plant biology. Its evolution has been crucial for the domestication of many crop species and their dissemination into new climatic regions (Colledge and Conolly 2007; Izawa 2007; Blümel et al. 2015). It is highly heritable, and the gene regulatory network controlling flowering time is very well described, making it an excellent trait to combine quantitative genetics and functional genomics. The impact of environmental cues on flowering time is well documented in the model plant Arabidopsis thaliana where a study (Li et al. 2010) identified SNPs that can explain up to 45 of the phenotypic variation of flowering time in a large panel of natural accessions. In sunflower, GWAS are more recent: Fusari et al. (2012) on disease resistance, and Nambeesan et al. (2015) on branching performed their GWAS with data collected on inbred lines, whereas Cadic et al. (2013) studied the genetic control of FT in a panel evaluated in 15 environments as hybrids.
Many crops, such as maize, sunflower and winter oil seed rape, are cultivated as hybrids. Hybrid vigor, or heterosis, was first observed by Kolreuter (1766). Genetic mechanisms underlying heterosis have been suggested, but their relative importance is not clearly elucidated (Lamkey and Edwards 1999). Different hypotheses including dominance (Bruce 1910; Jones 1917), overdominance (Crow 1948), and subsequently epistasis have been proposed (Williams 1959). Most GWAS models have been designed to consider only the additive effects of markers. Several studies have shown that non-additive effects constitute a major part of the variation of complex traits. These studies consider the intra-locus effects (Gengler et al. 1997; Norris et al. 2010), namely dominance, or inter-locus effects called epistasis (Huang et al. 2012; Mackay 2014). The work of Yang et al. (2014) on corn showed an increase in the proportion of heritability, explained because the model considered the dominance, thus allowing a better overview of heterosis. Mackay (2014) also stated that epistasis might be linked to missing heritability and small additive effects. Before them, Zhou et al. (2012) demonstrated on rice hybrids that the accumulation of multiple effects, including dominance and overdominance, might partially explain the genetic basis of heterosis. In human genetics, it has also been shown that models considering non-additive intra-locus effects yield new information, as in the case for the study by He et al. (2015), which found three new quantitative trait loci (QTLs) associated with kidney weight, compared to additive models. In contrast, Tsepilov et al. (2015) showed in humans that it is preferable to use non-additive effects only for traits where the non-additive function is known because additive models already capture a small part of the non-additive variability.
Mixed models are among the methods used to perform association analysis. They take into account the dependence between individuals by introducing a covariance structure for the genetic value of each individual and was proposed by Yu et al. (2006). The main drawback of the mixed model is its computational burden. So, new methods were proposed to accelerate the algorithm speed, EMMA (Kang et al. 2008) that avoid redundant matrix calculation, EMMAX (Kang et al. 2010) that is an approximation method with the ability to handle a large number of markers and finally GEMMA (Zhou and Stephens 2012) that is exact and efficient. All these methods are based on single-locus tests, but the traits can be controlled by many loci, with broader effects, and these models do not yield a good estimate of the markers effects in this case.
The identification of causal polymorphisms with the adjustment of more than one polymorphism at a time is complicated by the presence of linkage disequilibrium. Several multi-locus approaches have been proposed, including penalized regressions (Hoggart et al. 2008), Lasso (Yi and Xu 2008; Wang et al. 2011; Waldmann et al. 2013), and even the elastic net (Waldmann et al. 2013). Segura et al. (2012) proposed a regression method with inclusion by forward selection. This method involves EMMAX that reassesses the genetic and residual variances at each step of the algorithm. An assessment of the model quality, based on a selection criterion, is then performed.
The aim of our study was to evaluate different GWAS models that take dominance into account to detect associations in a hybrid panel and patterns of genetic control putatively involved in heterosis. For this purpose, we used the sunflower and flowering time as an example of the genetic control of complex traits, and we performed this study in a variety of environments to introduce realistic phenotypic variability. Several models involving intra-locus non-additive effects that are appropriate for a GWAS were tested. We sought to compare these models and conventional additive models of GWAS based on a multi-locus method similar to the one reported in Segura et al. (2012).
Materials and methods
Dataset collection
We collected data on the flowering time of sunflower (Helianthus annuus L.) from various French experiments conducted in 2013 by private partners (Biogemma, Caussade Semences, Maisadour Semences, RAGT2n, Soltis, Syngenta France) and by the French National Institute for Agricultural Research (INRA) as part of the SUNRISE project. Five experimental sites in different environments of regions in Southwestern France were planted with different hybrids from a set of 452 hybrids (between 303 and 444 hybrids per environment). Hybrids for this study were obtained by crossing 36 males and 36 females in an incomplete factorial design. They were chosen so that every parent was represented equivalently in the hybrid population (between 12 and 15 hybrids per parent).
In each environment, each measure of flowering time corresponded to one plot, planted with individuals of a single genotype. Each plot varied from 10 to 18 m depending on the environment, and the plant density (corresponding to the number of plants per m) was 5.8 on average and varied from three to eight plants per m. Flowering time was recorded when 50 of the plants in a plot were flowering and was then converted into degree days since the sowing date relative to the base 4.8 C, using the mean daily air temperature measured at each location.
Genotyping data
SNP genotyping was performed in the same way as in Badouin et al. (2017), but here using an Illumina type assembly. This work allowed us to obtain genotyping data from the 72 parents on 2,204,423 SNPs that were coded depending on the allele that a parent line could transmit to its descendants: 0, 1, or missing (0 for the XRQ allele, 1 for the variant). XRQ is the line used for the reference genome, described precisely in Badouin et al. (2017). The genotyping data were imputed by genomic scaffolds by means of BEAGLE (Browning and Browning 2009). Nevertheless, this step of the imputation of missing data created some redundancy among SNPs (SNPs are in complete linkage disequilibrium). Maintaining the redundancy for further GWAS analyses increases the computational burden. In addition, redundancy included in the calculation of the relatedness between hybrids tends to give more weight to regions containing many redundant markers, decreasing the power in these regions (Rincent 2014). Redundant SNPs were therefore discarded. One last filter on minor allele frequency (MAF) was implemented. SNPs with MAF (calculated for parent genotypes before imputation) less than 0.1 were discarded. A total of 478,874 non-redundant polymorphic SNPs were finally retained for various subsequent analyses. The genotypic data of hybrids were deduced from the genotypic data of the parents and coded as 0, 1, or 2 for homozygous XRQ and heterozygous and variant homozygous, respectively. In addition, the male and female origin of alleles was recorded for heterozygous SNPs.
Phenotype adjustment
Data were first adjusted using a linear model including two spatial fixed factors (line and column numbers in the field), a replicate fixed factor if necessary, an independent random genetic factor, and the residual error.
GWAS
The analyses were performed using a multi-locus approach with forward selection as proposed by Segura et al. (2012). This method is based on inclusion (at every step) of the SNP with the smallest p-value as a fixed regressor in a model that contains a random polygenic effect, as in classic GWAS model of Yu et al. (2006). The polygenic and residual variances are re-evaluated at each step, and a new scan of the remaining genome is performed. The more integrated the regressors in the model, the lower is the variance attributed to the random polygenic term. The forward selection analysis stops when the proportion of variance explained by this polygenic effect is close to zero. Five models were compared to find chromosomal regions linked to flowering time. These models have been coded and adapted for non-additive models, based on the MLMM code written by Segura et al. (2012), with ASReml-R (Butler et al. 2009). All scripts are available on request.
Two additive models: and models
The first model, as described in Segura et al. (2012), takes into account only the additive effect of markers. Let denote the adjusted phenotype of hybrid i. Then the additive model is
where is the centered genotype (coded as XRQ allelic dose) of the ith hybrid at the lth marker locus; is the additive effect of the lth locus; denotes the random polygenic effect; and is the residual error. Let and be vectors (, ) and (, ), respectively, and then , , where is a kinship matrix (relations among hybrids), and and are polygenic and residual variances, respectively.
One simple way to calculate the relatedness between hybrids based on molecular markers is to consider the proportion of shared alleles between two individuals, also called alike in state (AIS) relatedness.
The formula for biallelic markers (Maenhout et al. 2009) is
where L is the total number of markers, and are the vectors of genotypes for and (length of L, coded as XRQ allelic dose), and denotes a vector of two. The use of this formula for relatedness between hybrids does not consider haplotypic phases. However, haplotypic phases are known in our factorial design. Accordingly, we consider the AIS between the parents and known haplotypic phases to calculate the relatedness between hybrids. Thus, the AIS kinship that was used in the additive model designated the model was calculated as the average AIS between the respective parents of hybrids.
The other relationship matrix, used in the additive model designated the model, is equivalent to the unscaled kinship matrix described by VanRaden (2008):
where is the centered matrix of the hybrid genotypes.
The additive and dominant model: AD model
A model including additive and dominant effects of SNP markers as proposed by Su et al. (2012) was studied next. The model is
where is the centered genotype of the ith hybrid at the lth marker locus; is defined later; is the additive effect of the lth locus; is the dominance effect of the lth locus; and denotes error. is the random additive effect i, and is the random dominant effect i. Let , , and denote vectors (, ), (, ), and (, ), respectively, and then , , , where is the additive kinship matrix; is the dominance kinship matrix; and , and are additive, dominance and residual variances, respectively. as in the model, and where ; L is the number of loci; n denotes the number of hybrids; and
where is the XRQ allelic frequency at locus l within the parental population that is equal to the XRQ allelic frequency at locus l within the hybrid population under Hardy–Weinberg assumptions.
The part of additive variance used in the forward selection algorithm as a stopping criterion was defined in MLMM (Segura et al. 2012) by . To generalize the stopping criteria for the AD model, we used the ratio .
The models with female and male effects: FM and FMI model
These models include the male and female effects of SNP markers. The last also includes the interaction between the male and female effect. Let denote the adjusted phenotype of hybrid obtained when the female line f was crossed with the male line m, and then the model is
where is the centered (0 or 1) allele transmitted by the female f at the lth marker locus; is the centered (0 or 1) allele transmitted by the male m at the lth marker locus; ; is the female effect of the lth locus; is the male effect of the lth locus; and is the female–male interaction effect of the lth locus. , , and are the random effects of female f, male m, and their interaction, respectively, and denotes error. Let , , , and denote vectors (, ), (, ), (, ; ), and (, ; ), respectively, where and are the numbers of females and males, respectively. , , , where is the kinship matrix for the female; is the kinship matrix for the male; is the kinship matrix for the interaction between the male and female; and , , and are the female, male, female by male interaction, and residual variances, respectively. and as in the model but now using the centered matrix of transmitted alleles, is the Hadamard product between and , and .
The stopping criterion of the algorithm was defined by the ratio and for the FM and the FMI model, respectively.
Model selection and detected SNP estimation
The main problem of the multi-locus analysis is how much to integrate the SNPs into the model. BIC (Bayesian information criterion), which is generally used, is not strict enough for model selection in large model space (Chen and Chen 2008). Accordingly, eBIC (extended Bayesian Information Criterion), an extension of BIC, was developed (Chen and Chen 2008). It penalizes the BIC calculation by taking into account the number of possible models for a given number of regressors in the model using mathematical combination, also known as the binomial coefficient. For our models, the total and the given numbers of regressors used in mathematical combination depend on the SNP numbers and SNP modeling and are as follows:
where L is the total number of SNPs; is the number of variance components other than residual variance in the model; is the given number of SNPs in the model; and is the mathematical combination of among .
One way to choose the best is to find k, so that and then to assume (Chen and Chen 2008).
To calculate the effects of SNPs selected by eBIC, the model FMI, which is the most complete model, was used. It was composed of all eBIC-selected SNPs. Tukeys test of mean comparison was then performed to analyze the significance of differences among the four genotypic classes (00, 01, 10, and 11).
Linkage disequilibrium
Linkage disequilibrium was studied to compare and pool the discovered SNPs among models and environments. It was calculated between all pairs of SNPs selected by eBIC, using the classic (squared Pearson’s correlation) of the hybrid parent genotypes (i.e., SNP correlation of 36 males and 36 females). The significance level of linkage disequilibrium was found by randomly sampling independent SNPs. A total of 10,000 random pairs of SNPs (from 478,874) belonging to different chromosomes were processed. The significance threshold was computed as the quantile of the 10,000 distribution. We therefore focused on linkage disequilibrium values higher than this threshold of 0.155.
QTL definition
The use of QTLs instead of SNPs allows us to identify regions of interest rather than specific loci. A QTL is defined as a group of SNPs located on the same chromosome with linkage disequilibrium greater than the predefined significance threshold (0.155, which is the quantile of the 10,000 distribution of random independant SNP pairs), or an isolated SNP associated with a trait without the above characteristics. Since the 13EX03 and 13EX04 environments were not properly randomized, isolated SNPs from these environments were removed from the study.
For functional analysis, one SNP per QTL was selected as representative of the QTL. This choice was made based on the test p-value in an SNP by SNP model FMI. If a given SNP was associated with a trait in several environments, one p value per environment was calculated, and the minimal p-value was assigned to the SNP. The SNP ultimately representing the QTL is the one with the lowest p-value.
Results
Phenotypic data analysis
The period from sowing date to flowering time was measured in various environments. The flowering time in each environment was assumed to be a separate trait. Genotypic variance differed significantly from zero in all environments. The proportion of variance explained by genotypes (usually defined as broad sense heritability) ranged from 0.78 to 0.94 (Table 1). The proportion of variance explained by females (between 0.29 and 0.40) and males (between 0.34 and 0.40) is similar or slightly higher for males, particularly for the environment 13EX02.
Table 1.
Summary of part of variances
| 13EX01 | 13EX02 | 13EX03 | 13EX04 | 13EX06 | |
|---|---|---|---|---|---|
| 0.86 | 0.79 | 0.94 | 0.91 | 0.88 | |
| 0.35 | 0.29 | 0.40 | 0.36 | 0.35 | |
| 0.34 | 0.37 | 0.40 | 0.39 | 0.38 |
For each environment (13EX01 to 13EX06), the proportion of phenotypic variance explained by genotypes (), by females () and by males (), are presented
Flowering time is not known as a highly heterotic trait. However, some hybrids exhibited a visible heterotic phenotype, when looking at the hybrid performances according to their parents (Figure S1), particularly in 13EX01 and 13EX02 environments. As examples, the hybrid resulting from the cross between the female SF301 and the male SF336 on 13EX01 and that from the cross between the female SF217 and the male SF324 on 13EX02, showed later flowering time than expected if the trait was governed only by additive behavior. Dominance was recorded in Table 2, by comparing the hybrid performances to the general combining ability (GCA) of their parents. Between 3 and 13 hybrids were dominant (i.e., the phenotypic value of a hybrid was more or less than two standard deviations from the average of its parents).
Table 2.
Number of hybrids with heterotic phenotype
| Env | Nb of hybrids | 2 SD | Dom+ | Dom− |
|---|---|---|---|---|
| 13EX01 | 303 | 54.5 | 9 | 9 |
| 13EX02 | 444 | 56.13 | 13 | 7 |
| 13EX03 | 424 | 71.19 | 11 | 3 |
| 13EX04 | 428 | 69.48 | 6 | 10 |
| 13EX06 | 430 | 61.17 | 13 | 6 |
For each environment (13EX01 to 13EX06), hybrids phenotyped, the value of two standard deviation (2 SD) and hybrids with phenotypic value more (Dom+) or less (Dom−) than two standard deviations from the average of their parents were quantified
The correlations among environments are high (Figure S2), ranging from 0.68 to 0.85. These correlations were calculated only on common hybrids between the tested environments, i.e., between 297 and 425 hybrids. Environment 13EX02 correlates with the others the least, with correlation coefficients between 0.679 and 0.697. This result can be explained by the fact that the sowing date for this environment was 7–20 days after the other sowing dates. In addition, measurement in this environment was performed less regularly. Despite the good correlation among environments, we analyzed each one independently to capture environment-specific associations.
SNPs associated with the trait
Table 3 shows the number of associated SNPs in each model by environment. For analysis involving models that consider only additive effects ( and ), the number of associated SNPs ranges between two for model in environment 13EX01, for example, and eight for the same model in environment 13EX03. In the analysis with model , the number of SNPs associated with the trait is greater or equal to the number of SNPs in model for all environments except 13EX01. For association analysis involving models other than additive ones (AD, FM, and FMI), the eBIC selection only retains a single SNP, despite the fact that the non-additive parts of variance are significant for some environments (Table S1). In total, among all models and all environments, 31 unique SNPs are associated with the flowering time.
Table 3.
Number of SNPs associated with flowering time selected by the forward approach and eBIC per environment and per model
| 13EX01 | 13EX02 | 13EX03 | 13EX04 | 13EX06 | |
|---|---|---|---|---|---|
| 2 | 3 | 8 | 4 | 6 | |
| 4 | 3 | 5 | 4 | 2 | |
| AD | 1 | 1 | 1 | 1 | 1 |
| FM | 1 | 1 | 1 | 1 | 1 |
| FMI | 1 | 1 | 1 | 1 | 1 |
The results for additive models with different kinships ( and ) and non-additive models including dominance (AD), female and male effects (FM), and female, male, and their interaction effects (FMI) are presented in five environments (13EX01–13EX06)
The MLMM approach selects a single SNP, i.e., the most associated one, to explain the effect of the causal polymorphism in this genomic region. However, several SNPs could be in LD with the causal polymorphism and different sources of errors (phenotypic and genotypic), and missing data could lead to the selection of different SNPs to explain the same causal polymorphism in our different experiments. Therefore, we grouped the SNPs to define QTLs and refer to regions rather than specific positions. This grouping was achieved using linkage disequilibrium between SNPs and positions on the sunflower genomic reference sequence (Badouin et al. 2017).
Estimation of linkage disequilibrium (LD)
All SNP pairs with (squared Pearson’s correlation) above 0.155 were considered to be in linkage disequilibrium. This significance threshold was defined as the quantile of the distribution obtained for 10,000 randomly sampled pairs of independent SNPs.
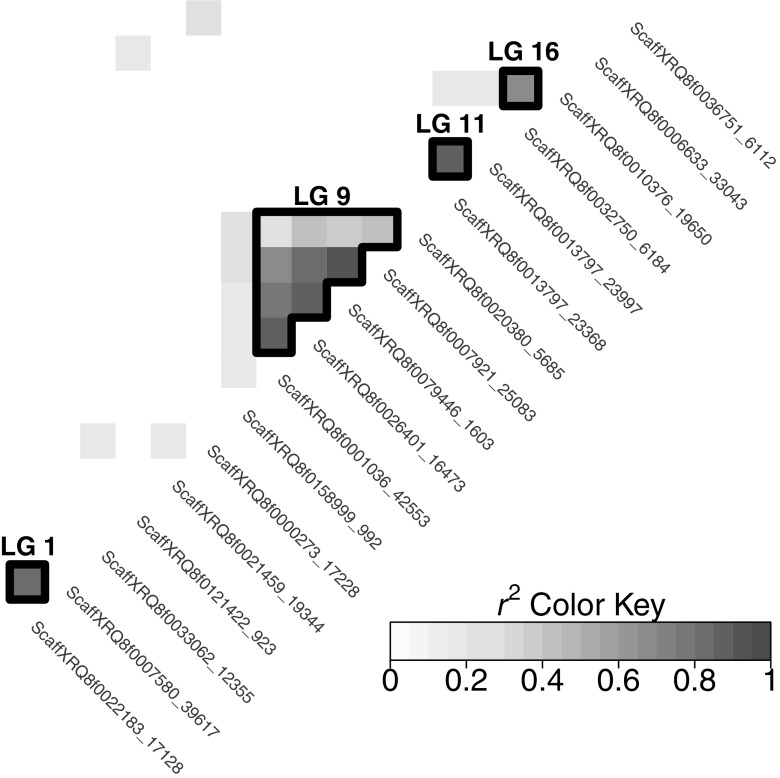
We studied the linkage disequilibrium between the SNPs selected by eBIC for all models and environments. Figure 1 illustrates (according to the physical positions of SNPs in the XRQ reference genome) only disequilibria greater than the significance threshold of 0.155. Eighteen SNPs of the 31 SNPs selected by eBIC for all models and environments are in desequilibrium with another. Pairs of SNPs located on chromosome LG01, LG11, and LG16 are in strong LD. An LD block is located on chromosome LG09 ( between 0.29 and 0.93). One SNP in disequilibrium with this group is itself located on chromosome LG07. These LDs correspond either to long-range disequilibria that can be caused by imperfect positioning of contigs in the reference genome or to the limited size of our parental population. With the statistical risk at (it should be reduced to take into account the multiplicity of LD tests between all pairs of discovered SNPs), we obtained a threshold of 0.155, which is slightly lower than the linkage disequilibrium thresholds used in other association studies on the sunflower [ 0.2 reported by Cadic et al. (2013) and Nambeesan et al. (2015)]. In total, this approach allowed us to build 13 associated regions (QTLs) for flowering time on 11 chromosomes.
Fig. 1.
Heatmap of linkage disequilibria between SNPs associated with the flowering time, among all environments and models. Only linkage disequilibria above the significance threshold of 0.155 were represented (18 SNPs of the 31 SNPs selected by eBIC for all models and environments are in linkage desequilibrium). Black lines highlight linkage disequilibria between SNPs on the same chromosome. The linkage group (LG) is indicated above a group of interest in black
QTL description
Groups of five or two SNPs in LD, together with single SNPs define the QTLs presented in Table 4. Figure 2 represents for each environment and for each model the positions of the detected SNPs. It is noteworthy that four of the five SNPs defining FT09.199 were obtained with non-additive association models, and this QTL was detected on four of five environments. Similarly, the FT11.47 region was only detected by non-additive models, but only on one environment. It is the only QTL that would not have been detected with the conventionally used additive models. The FT15.102 region was detected by the model taking into account female and male effects, but mainly by both additive models in all environments (Fig. 2 and Table S2). The remaining ten QTLs were detected by additive models only and tend to have higher p values than non-additive QTLs (Table 4 and Figure S3 to Figure S7). Among these ten QTLs, seven QTLs were specific to one environment and three were detected on two environments.
Table 4.
List of QTLs associated with flowering time
| QTL | SNP | LG | Position | MAF | Models | p value |
|---|---|---|---|---|---|---|
| FT09.199 | ScaffXRQ8f0001036_42553 | 9 | 198,931,169 | 0.26 | AD, FMI | 1.84 |
| ScaffXRQ8f0026401_16473 | 9 | 199,047,735 | 0.32 | AD, FMI, FM | 8.67 | |
| ScaffXRQ8f0079446_1603 | 9 | 199,131,966 | 0.33 | AD, FMI | 1.86 | |
| ScaffXRQ8f0007921_25083 | 9 | 199,145,681 | 0.29 | 6.14 | ||
| ScaffXRQ8f0020380_5685 | 9 | 201,493,137 | 0.24 | AD, FMI | 3.57 | |
| FT11.47 | ScaffXRQ8f0013797_23368 | 11 | 47,534,503 | 0.42 | AD | 4.16 |
| ScaffXRQ8f0013797_23997 | 11 | 47,535,132 | 0.39 | FM, FMI | 3.62 | |
| FT16.167 | ScaffXRQ8f0010376_19650 | 16 | 167,723,083 | 0.39 | , | 2.76 |
| ScaffXRQ8f0032750_6184 | 16 | 167,689,531 | 0.42 | 6.74 | ||
| FT01.98 | ScaffXRQ8f0007580_39617 | 1 | 98,035,404 | 0.17 | , | 6.77 |
| ScaffXRQ8f0022183_17128 | 1 | 91,634,676 | 0.21 | 2.40 | ||
| FT15.102 | ScaffXRQ8f0000770_77572 | 15 | 102,863,872 | 0.26 | , , FM | 1.91 |
| FT02.78 | ScaffXRQ8f0070840_1738 | 2 | 78,884,560 | 0.11 | , | 3.55 |
| FT17.184 | ScaffXRQ8f0036751_6112 | 17 | 184,825,665 | 0.18 | , | 4.64 |
| FT05.208 | ScaffXRQ8f0006894_28213 | 5 | 208,225,977 | 0.21 | 1.42 | |
| FT04.144 | ScaffXRQ8f0065196_696 | 4 | 144,357,532 | 0.36 | 1.34 | |
| FT07.34 | ScaffXRQ8f0001757_13384 | 7 | 34,580,910 | 0.11 | 4.15 | |
| FT17.13 | ScaffXRQ8f0006633_33043 | 17 | 13,852,550 | 0.39 | 3.10 | |
| FT04.74 | ScaffXRQ8f0021459_19344 | 4 | 74,011,326 | 0.19 | 1.65 | |
| FT13.190 | ScaffXRQ8f0023382_14615 | 13 | 190,953,163 | 0.12 | 8.91 |
For each QTL, the following information on the detected SNP is presented: chromosome (LG), position (bp), minor allele frequency (MAF), GWAS model: additive with different kinships ( and ) and non-additive including dominance (AD), female and male effects (FM), and female, male and their interaction effects (FMI), and p-values calculated in the FMI model, incorporating only the detected SNP. For each QTL composed of several SNPs, the SNP with the smallest p-value is highlighted in bold
Fig. 2.
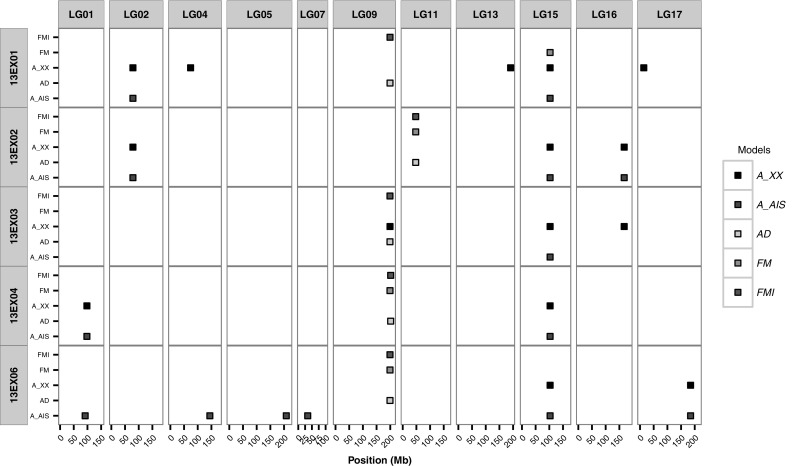
Positions of SNPs associated with the flowering time per environment and model. For each environment (13EX01–13EX06) and for each model (FM, FMI, , AD and ), the positions (in Mb) of the detected SNPs are represented by a square. The squares are colored in accordance with the model in which they were detected. Only chromosomes (LG) with detected SNPs are represented
QTL effects
We characterized the effects of the SNPs detected in both the additive and non-additive models. Regarding QTLs detected by the additive models, the majority of SNPs have a clearly additive profile similar to Fig. 3a. However, for some additive SNPs, Tukey’s mean comparison test did not separate the genotypes in three significantly different classes, certainly because of a lack of power.
Fig. 3.
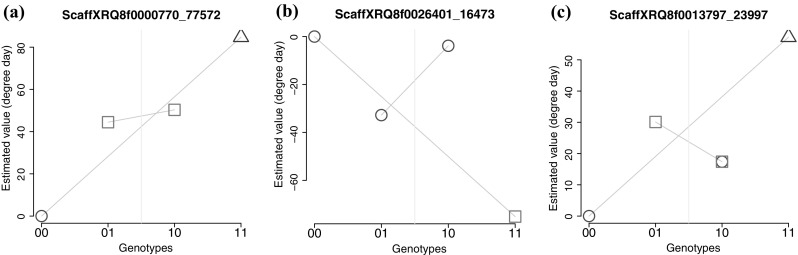
Effects of SNPs on flowering time for the four genotypic classes. a Example of an additive SNP. b SNP discovered with non-additive model and with a dominant trend for one allele. c SNP discovered with non-additive model and with an additive trend. 00 and 11 correspond to homozygous genotypes, 10 to the heterozygous genotype that received allele 1 from the female parent, and 01 to the heterozygous genotype that received allele 1 from the male parent. Each symbol indicates membership in a specific class in Tukey’s mean comparison test with a statistical risk. Two superimposed symbols indicate that the Tukey’s mean comparison test failed to determine a single class for the genotype
The majority of QTLs detected using non-additive models have a profile similar to Fig. 3b, with a dominant trend for one allele (reference allele of inbred line XRQ for the male in the example). Two significantly different classes in the mean comparison test, separating one homozygous genotype from the other genotypes, is expected for a dominant allele. Figure 3c illustrates SNP profiles that are more difficult to interpret. Such profiles could be due to slight dominance of the XRQ allele in males or more probably to an additive SNP and insufficient power of Tukey’s test.
QTL annotations
For each QTL, the SNP with the lowest p-value in the model FMI was selected to represent the region. All redundant SNPs were excluded from the GWAS analysis, but in terms of the functionality of the gene, information on the location of redundant SNPs is important. SNPs redundant with SNPs that are representative of a QTL were therefore recovered and analyzed in the same way as other SNPs. The results of this analysis are presented in Table 5. All SNPs redundant with the referent SNP of FT09.199 are also located on chromosome LG09 at positions very close to each other (within a 61 kb interval). Two genes are present in this region, but none is known to be involved in flowering. Four QTLs are also located in the identified genes on chromosomes LG05, LG13, LG16, and LG17. These genes do not correspond to a flowering-related gene. One SNP located on chromosome LG17 is redundant with the referent SNP of FT11.47 and another SNP also on chromosome LG11. This situation may be due to the imperfect quality of the genome.
Table 5.
Genes underlying QTLs associated with flowering time
| QTL | LG | Position | Nearest gene | In.Out | DistToStart |
|---|---|---|---|---|---|
| FT01.98 | 1 | 98,035,404 | HanXRQChr01g0016411 | Upstream | −11,986 |
| FT02.78 | 2 | 78,884,560 | HanXRQChr02g0042521 | Downstream | 295,211 |
| FT04.74 | 4 | 74,011,326 | HanXRQChr04g0107731 | Upstream | −93,659 |
| FT04.144 | 4 | 144,357,532 | HanXRQChr04g0118011 | Downstream | 129,288 |
| FT05.208 | 5 | 208,225,977 | HanXRQChr05g0160261 | In | 81 |
| FT07.34 | 7 | 34,580,910 | HanXRQChr07g0191191 | Upstream | −651 |
| FT09.199 | 9 | 199,047,452 | HanXRQChr09g0272971 | In | 18,574 |
| 9 | 199,047,477 | HanXRQChr09g0272971 | In | 18,599 | |
| 9 | 199,047,735 | HanXRQChr09g0272971 | In | 18,857 | |
| 9 | 199,071,389 | HanXRQChr09g0272971 | In | 42,511 | |
| 9 | 199,109,369 | HanXRQChr09g0272981 | In | 5822 | |
| FT11.47 | 11 | 47,260,646 | HanXRQChr11g0330951 | Upstream | −81,868 |
| 11 | 47,535,132 | HanXRQChr11g0330981 | Upstream | −82,521 | |
| FT13.190 | 13 | 190,953,163 | HanXRQChr13g0424551 | In | 3214 |
| FT15.102 | 15 | 102,863,872 | HanXRQChr15g0487841 | Upstream | −44,505 |
| FT16.167 | 16 | 167,723,083 | HanXRQChr16g0528041 | In | 31,859 |
| FT11.47 | 17 | 175,837,528 | HanXRQChr17g0564111 | Upstream | −5234 |
| FT17.13 | 17 | 13,852,550 | HanXRQChr17g0537591 | In | 4839 |
| FT17.184 | 17 | 184,825,665 | HanXRQChr17g0565411 | Upstream | −13,145 |
One SNP per QTL was selected, and its redundancy, if applicable, was also analyzed. The table describes QTL name (QTL), chromosome (LG), position (Position), closest gene, location with respect to the closest gene (In.Out), and distance to the start of the closest gene (DistToStart)
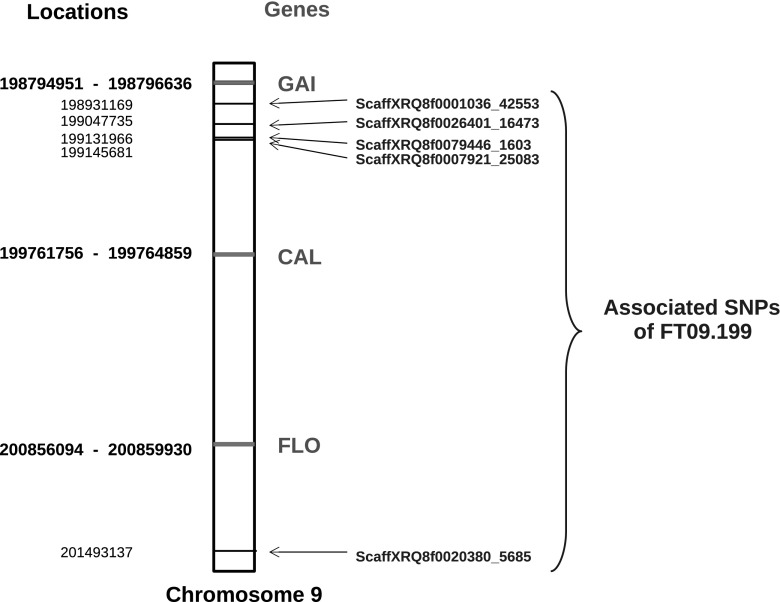
Few of the SNPs are located in genes, but three genes known to be involved in the flowering process are located on chromosome LG09. Figure 4 presents the positions of the associated markers and these three genes in FT09.199. GIBBERELLIC ACID INSENSITIVE (GAI, homologous to HanXRQChr09g0272901) is a gene involved in flowering time (Wilson and Somerville 1995), whereas FLORICAULA (FLO, homologous to HanXRQChr09g0273821) (Coen et al. 1990) and CAULIFLOWER (CAL, homologous to HanXRQChr09g0273361) (Bowman et al. 1993) are genes involved in flowering development. FT09.199 consists of four SNPs very close together (within a 214 kb interval) and a more distant SNP (at 2 Mb down chromosome LG09). The most interesting gene based on its function, namely GAI, is the closest of the four SNPs. This region was further examined based on the p-values of all SNPs in it. Figure S8 represents the p-values for all SNPs of the FT09.199 region and the three genes involved in the flowering. The presented p-values were calculated in the environment and with the model where the SNP of interest was discovered in association. It can be seen that the most significant associations are found in the region of the first four SNPs. With the FMI model and for the environments 13EX03 and 13EX06, we can see the SNP with low p-values downstream, i.e., between the two CAL and FLO genes.
Fig. 4.
Locations of genes involved in the flowering process, compared to locations of SNPs of FT09.199 located in the same region of the chromosome LG09. Gene and SNP positions are indicated in bold and normal font, respectively. For genes, the two positions correspond to the start and end of the gene
Discussion
In this study, we propose new GWAS models including non-additive effects. These models were developed to better model the biological factors involved in sunflower trait variability. Indeed, the modeling of intra-locus effects with a dominance component can capture part of heterosis (Larièpe et al. 2012; Reif et al. 2012), a phenomenon usually observed in sunflower hybrids (Cheres et al. 2000). In addition, the modeling of differences in male and female allelic effects takes into account the two sunflower breeding groups, for which divergence between the maintainer and restorer germplasm has previously been observed by Gentzbittel et al. (1994). As in the common additive GWAS model, there is a one-to-one correspondence, in these models between a non-additive fixed effect and its random effect. A correct model to test each QTL is a model that has known all causal QTLs of the trait genetic architecture and their location in the genome. Because the QTL locations are unknown, this perfect model is unknown, and a model assuming a QTL effect on each marker is considered. However, the number of parameters in this model is then larger than the number of observed individuals, and to address this issue the solution is to assume a normal distribution for the marker effects in linkage equilibrium with the tested locus . Then, as in the equivalence between the rrBLUP method (Endelman 2011) and the GBLUP method (VanRaden 2008), it leads to as many fixed effects as random effects, depending on the non-additive model used. The computational burden required for computing kinship matrices at each location is then lessened by using the same kinship matrices for all . As shown by Rincent (2014), having different kinship matrices improves the power of GWAS analyses, since it avoids absorbing a part of the signal roughly proportional to the linkage disequilibrium in the region of when testing at location .
The additive model taking into account the phase in the kinship’s computation does not improve the power of the QTL detection, compared to the additive model conventionally used. The difference between the two additive models lies in the kinship matrix computation: one is the usual (VanRaden 2008) matrix and the other an AIS-like matrix that takes into account known marker phases in hybrids. Both models detected the greatest number of associated SNPs and of QTLs in common. Indeed, five QTLs are found associated using both additive models and, in particular, FT15.102 was detected in all environments. The QTLs that differ between these models have higher p values and therefore are less strongly associated with the phenotype. Overall, the two additive models yield coherent results, especially on strongly associated QTLs. Strandén and Christensen (2011) demonstrated that the use of a VanRaden (2008) or AIS relatedness matrix gives the same prediction of additive genetic values in the GBLUP genomic selection model, and in particular proved that both matrices give the same REML estimates of random variance components. Therefore, the Wald tests performed in the GWAS forward approach are identical for the two relatedness matrices. Although we used more information in our AIS-like matrix because we integrated the known marker phases, we did not obtain a power improvement in QTL detection, as could be expected.
One QTL, FT09.199, is the most interesting region in our study. FT09.199 was found to be associated with flowering time with four models out of five. This region is located on chromosome LG09, and this chromosome was also highlighted by Cadic et al. (2013). In their study, the region is found to be associated in six different environments (i.e., combinations Sites Years). In addition, three genes [GAI (Wilson and Somerville 1995), FLORICAULA (Coen et al. 1990), and CAULIFLOWER (Bowman et al. 1993)] known to be involved in flower development are also located on chromosome LG09. It is surprising that none of our results falls exactly into these three genes, but FT09.199 is near GAI. It is likely that the causal polymorphism could be close and in strong linkage disequilibrium with the associated SNPs without being located exactly at the same position. A QTL confidence interval around FT09.199 would be useful to estimate the region where the causal locus should be located. Hayes (2013) proposed a method based on the difference of QTL positions within the region of interest detected in two random subsamples; this method could be applied to our QTL.
The most interesting region on LG09 was emphasized by non-additive models. Indeed, three models were tested to provide additional association to GWAS results usually based on additive effect (Yu et al. 2006; Segura et al. 2012). This interesting region is indicated by five SNPs, among which a single SNP was detected by an additive model. The non-additive modeling results increase the reliability of this region through the identification of SNPs very close to the SNP identified using an additive model. Moreover, four SNPs out of five were detected with the FMI model, which is the most complex. The usefulness of non-additive models is also illustrated by FT11.47 (on chromosome LG11), since this QTL was only detected with non-additive models despite having a strong impact on flowering time, as illustrated by its effects and p-values in the FMI model. In addition, models AD and FMI, which include intra-locus interaction by modeling dominance or parental allelic interaction, both found the most strongly associated regions indicated by FT09.199 and FT11.47. The first exhibits a clear deviation from an additive behavior, in contrast to the latter, for which an additive behavior cannot be rejected. FT11.47 was not found by additive models, because there is linkage disequilibrium between it and the strong FT16.167 on LG16 detected by additive models. In our forward detection procedure of the additive models, this phenomenon led to the addition of FT16.167 first, which likely decreased the signal of FT11.47 and prevented its detection. Performing GWAS with different models allowed us to increase both the number of associated QTLs and the confidence in the detected regions. Non-additive models can highlight regions with non-additive behavior even for a trait such as flowering time, which is notably genetically additive (Miller et al. 1980; Roath et al. 1982).
The extended BIC criterion used to select associated SNPs has limitations. In our procedure, the eBIC used to choose non-additive models had two major drawbacks that certainly decreased the number of QTLs detected by these models and thus limited their usefulness. eBIC is an extension of BIC suitable for handling the so-called “high-dimension issue” resulting from fewer observations than possible regressors to be put in the model. A penalization term that depends on the number of possible models formed with a given number of regressors is added to BIC in the eBIC calculation (Chen and Chen 2008). eBIC was established for additive regressor models, and therefore we had to adapt it to the non-additive models AD, FM, and FMI. We generalized eBIC to non-additive models by computing the penalization term as if all possible models formed with a given number of regressors were analyzed. Nonetheless, it is clear that all possible models are not analyzed during the forward selection process. Indeed, each SNP selected by the algorithm is added to the current model with all its modeling effects. The dominant part of an SNP cannot be added without the additive part, if we take the AD model as an example. The number of possible models should have been reduced to take this constraint into account and the penalization term is therefore too high and not completely suitable for non-additive models. Furthermore, we calculated a criterion to make a model choice among models that do not share the same number of fixed effects (a new SNP is incorporated at each iteration of the forward algorithm). The restricted maximum likelihood (REML) used for this calculation [on the basis of the calculation of Segura et al. (2012)] is not the correct likelihood to use. The maximum likelihood (ML) should have been used instead of REML. Moreover, Gurka (2006) demonstrated that REML should incorporate the fixed effects using , where is the fixed effect design matrix, and showed by simulations that this added term in REML computation gave similar or better results than ML. The stringency of eBIC due to a too high penalization term and the absence of the term due to fixed effects may both explain why only a single SNP was selected by the non-additive models for each environment. Nevertheless, as it is more acceptable to exclude too many false negatives than to select too many false positives, we kept the eBIC for our model choice.
The study of the significant differences between genotypic effects highlighted FT09.199 as far from an additive profile, with homozygotes for the variant allele that flower earlier than the other genotypes. Eighty degree days separate variant homozygotes from XRQ allele homozygotes, i.e., a difference of nearly 6 days. This effect is very important regarding the observed variability of approximately 15 days in the multi-environment trials. Flowering time is an important agronomic trait that impacts crop yield, ecological fitness including adaptation to abiotic factors, and interaction with pollinators. Knowledge of the relative lengths of the period from sowing to flowering is particularly important for breeding yield (Tuteja 2012), as late hybrids accumulate more biomass than early hybrids, and this advantage can lead to a higher yield (Cadic 2014). For a maximal dry matter yield, all parts of the plant need to develop. This morphology corresponds to late-flowering genotypes (Gallais et al. 1983). The aim of breeders is to find genotypes with the best performance; so regarding the selection of sunflower lines, studies tend to select late lines. Precocity is linked to yield, and therefore the variability of the effect of SNP associated with the flowering time for different genotypes is of interest.
No clear difference between models differentiating female and male effects (FM and FMI models) and the other models was observed. The two breeding pools of sunflower (maintainers and restorers of male sterility) have undergone neither the same trait improvement nor the same selection pressure (Mandel et al. 2011). It is therefore expected that modeling different effects for each parental allele, as in FM and FMI models, will yield different results from other models. However, this expected difference in QTL detection is not obvious in our study, and only a single QTL was detected exclusively by the FM and FMI models. A lack of differentiation between the two breeding pools in this study compared to Mandel et al. (2011) or the too small number of non-additive QTLs detected because of eBIC could explain this result. Furthermore, even if there are highly differentiated regions between pools, they may not be involved in flowering time variability, as branching and restorer of cytoplasmic male sterility are located on chromosomes LG10 and LG13.
Intra-environment GWAS in a multi-environment trial allows to reveal generalist QTLs whose action does not depend on the environment. In our study, we detected five generalist QTLs revealed despite the disturbance in observed hybrid panel and in trait variation due to different experimental sources (location, climate, soil, cultural practices, and biotic stress). We also detected height QTLs, found associated in only one environment. These two types of QTLs are usually observed in multi-environment GWAS, as in sunflower (Cadic et al. 2013) or in Brassica napus (Li et al. 2015); however, it is difficult to claim that QTL found in a single environment is environment specific, as power of GWAS could be different from an environment to another, thus leading to detection of less strong association signal. Naturally, the confidence is greater for generalist QTLs, and a region indicated by several SNPs, when they exist, could help to define a confidence region for the underlying causal locus.
Non-additive effects, including dominance or overdominance, have been suggested as underlying heterosis. The modeling of non-additive effects in our models captured part of the heterosis observed in hybrids. This study shows the added value of non-additive modeling of allelic effects, and thus the importance of taking into account heterosis, to identify genomic regions controlling traits of interest for sunflower hybrids.
Author contribution statement
FB performed statistical analysis NB, M-CB, GM, EB-M, and NP contributed to the DNA sample collection and data production. JG, genome assembly LL, GF performed bioinformatic analyses SC. JG provided bioinformatic ressources. FB and BM developed the statistical pipeline. NBL, SM, and JG designed experiments and coordinated the project. FB, NBL, and BM wrote the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
All these analyses would not have been possible without the experimental data, for which we thank all partners of the “SUNRISE” project of the French National Research Agency (ANR-11-BTBR-0005, 2012-2019): Biogemma, Caussades semences, Maisadour semences, RAGT 2n, Soltis, Syngenta, and Terres Inovia.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest
Footnotes
This work was supported by the SUNRISE project, and we thank all the partners.
Electronic supplementary material
The online version of this article (doi:10.1007/s00122-017-3003-4) contains supplementary material, which is available to authorized users.
References
- Badouin H, Gouzy J, Grassa CJ, Murat F, Staton SE, Cottret L, Lelandais-Brière C, Owens GL, Carrère S, Mayjonade B, et al. The sunflower genome provides insights into oil metabolism, flowering and asterid evolution. Nature. 2017;546:148–152. doi: 10.1038/nature22380. [DOI] [PubMed] [Google Scholar]
- Blümel M, Dally N, Jung C. Flowering time regulation in cropswhat did we learn from arabidopsis? Curr Opin Biotechnol. 2015;32:121–129. doi: 10.1016/j.copbio.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Alvarez J, Weigel D, Meyerowitz EM, Smyth DR. Control of flower development in arabidopsis thaliana by apetala1 and interacting genes. Development. 1993;119(3):721–743. [Google Scholar]
- Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Gene. 2009;84(2):210–223. doi: 10.1016/j.ajhg.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A. The mendelian theory of heredity and the augmentation of vigor. Science. 1910;32(827):627–628. doi: 10.1126/science.32.827.627-a. [DOI] [PubMed] [Google Scholar]
- Butler D, Cullis BR, Gilmour A, Gogel B. Asreml-r reference manual. Brisbane: Department of Primary Industries and Fisheries; 2009. [Google Scholar]
- Cadic E (2014) Recherche de facteurs gntiques impliqus dans l’laboration du rendement sous contrainte hydrique chez le tournesol helianthus annuus par gntique d’association et analyse de liaison dans une population recombinante. Ph.D. thesis, Universit de Toulouse
- Cadic E, Coque M, Vear F, Grezes-Besset B, Pauquet J, Piquemal J, Lippi Y, Blanchard P, Romestant M, Pouilly N, et al. Combined linkage and association mapping of flowering time in sunflower (Helianthus annuus l.) Theor Appl Gene. 2013;126(5):1337–1356. doi: 10.1007/s00122-013-2056-2. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen Z. Extended bayesian information criteria for model selection with large model spaces. Biometrika. 2008;95(3):759–771. doi: 10.1093/biomet/asn034. [DOI] [Google Scholar]
- Cheres M, Miller J, Crane J, Knapp S. Genetic distance as a predictor of heterosis and hybrid performance within and between heterotic groups in sunflower. Theor Appl Gene. 2000;100(6):889–894. doi: 10.1007/s001220051366. [DOI] [Google Scholar]
- Coen ES, Romero J, Doyle S, Elliott R, Murphy G, Carpenter R. Floricaula: a homeotic gene required for flower development in antirrhinum majus. Cell. 1990;63(6):1311–1322. doi: 10.1016/0092-8674(90)90426-F. [DOI] [PubMed] [Google Scholar]
- Colledge S, Conolly J (2007) The origins and spread of domestic plants in Southwest Asia and Europe. Left Coast Press
- Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, Roses AD, Haines J, Pericak-Vance MA. Gene dose of apolipoprotein e type 4 allele and the risk of alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Crow JF. Alternative hypotheses of hybrid vigor. Genetics. 1948;33(5):477. doi: 10.1093/genetics/33.5.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endelman JB. Ridge regression and other kernels for genomic selection with r package rrblup. Plant Gen. 2011;4(3):250–255. doi: 10.3835/plantgenome2011.08.0024. [DOI] [Google Scholar]
- Ersoz ES, Yu J, Buckler ES (2007) Applications of linkage disequilibrium and association mapping in crop plants. In: Varshney RK, Tuberosa R (eds) Genomics-assisted crop improvement, Springer, pp 97–119
- Fusari CM, Di Rienzo JA, Troglia C, Nishinakamasu V, Moreno MV, Maringolo C, Quiroz F, Álvarez D, Escande A, Hopp E, et al. Association mapping in sunflower for sclerotinia head rot resistance. BMC Plant Biol. 2012;12(1):1. doi: 10.1186/1471-2229-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallais A, Vincourt P, Bertholleau JC (1983) Etude de critères de s élection chez le mais fourrage :héritabilités, corrélations génétiques et réponse attendue. Agronomie
- Gengler N, Van Vleck LD, MacNeil M, Misztal I, Pariacote F. Influence of dominance relationships on the estimation of dominance variance with sire-dam subclass effects. J Anim Sci. 1997;75(11):2885–2891. doi: 10.2527/1997.75112885x. [DOI] [PubMed] [Google Scholar]
- Gentzbittel L, Zhang YX, Vear F, Griveau B, Nicolas P. Rflp studies of genetic relationships among inbred lines of the cultivated sunflower, Helianthus annuus l.: evidence for distinct restorer and maintainer germplasm pools. Theor Appl Gene. 1994;89(4):419–425. doi: 10.1007/BF00225376. [DOI] [PubMed] [Google Scholar]
- Gurka MJ. Selecting the best linear mixed model under reml. Am Stat. 2006;60(1):19–26. doi: 10.1198/000313006X90396. [DOI] [Google Scholar]
- Hayes B. Overview of statistical methods for genome-wide association studies (GWAS) Methods Mol Biol. 2013;1019:149–169. doi: 10.1007/978-1-62703-447-0_6. [DOI] [PubMed] [Google Scholar]
- He Y, Li X, Zhang F, Su Y, Hou L, Chen H, Zhang Z, Huang L. Multi-breed genome-wide association study reveals novel loci associated with the weight of internal organs. Gene Sel Evol. 2015;47(1):1. doi: 10.1186/s12711-014-0081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggart CJ, Whittaker JC, De Iorio M, Balding DJ. Simultaneous analysis of all snps in genome-wide and re-sequencing association studies. PLoS Genet. 2008;4(7):e1000,130. doi: 10.1371/journal.pgen.1000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Richards S, Carbone MA, Zhu D, Anholt RR, Ayroles JF, Duncan L, Jordan KW, Lawrence F, Magwir MM, et al. Epistasis dominates the genetic architecture of drosophila quantitative traits. Proceed Natl Acad Sci. 2012;109(39):15,553–15,559. doi: 10.1073/pnas.1213423109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa T. Adaptation of flowering-time by natural and artificial selection in arabidopsis and rice. J Exp Bot. 2007;58(12):3091–3097. doi: 10.1093/jxb/erm159. [DOI] [PubMed] [Google Scholar]
- Jones DF. Dominance of linked factors as a means of accounting for heterosis. Proceed Natl Acad Sci. 1917;3(4):310–312. doi: 10.1073/pnas.3.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E. Efficient control of population structure in model organism association mapping. Genetics. 2008;178(3):1709–1723. doi: 10.1534/genetics.107.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HM, Sul JH, Service SK, Zaitlen NA, Kong SY, Freimer NB, Sabatti C, Eskin E, et al. Variance component model to account for sample structure in genome-wide association studies. Nat Genet. 2010;42(4):348–354. doi: 10.1038/ng.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolreuter J (1766) Jg 1761–1766. Vorliufige Nachricht von einigen das Geschlecht der Pflanzen betreffenden Versuchen und Beobachtungen Gleditsch, Leipzig
- Lamkey KR, Edwards JW (1999) Quantitative genetics of heterosis. In: Coors JG, Pandey S (eds) The Genetics and Exploitation of Heterosis in Crops. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, WI, pp 31–48
- Larièpe A, Mangin B, Jasson S, Combes V, Dumas F, Jamin P, Lariagon C, Jolivot D, Madur D, Fievet J, et al. The genetic basis of heterosis: multiparental quantitative trait loci mapping reveals contrasted levels of apparent overdominance among traits of agronomical interest in maize (Zea mays l.) Genetics. 2012;190(2):795–811. doi: 10.1534/genetics.111.133447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Long Y, Zhang L, Dalton-Morgan J, Batley J, Yu L, Meng J, Li M. Genome wide analysis of flowering time trait in multiple environments via high-throughput genotyping technique in Brassica napus L. PLoS One. 2015;10(3):e0119,425. doi: 10.1371/journal.pone.0119425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Huang Y, Bergelson J, Nordborg M, Borevitz JO. Association mapping of local climate-sensitive quantitative trait loci in Arabidopsis thaliana. Proceed Natl Acad Sci. 2010;107(49):21,199–21,204. doi: 10.1073/pnas.1007431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TF. Epistasis and quantitative traits: using model organisms to study gene–gene interactions. Nat Rev Genet. 2014;15(1):22–33. doi: 10.1038/nrg3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenhout S, De Baets B, Haesaert G. Marker-based estimation of the coefficient of coancestry in hybrid breeding programmes. Theor Appl Genet. 2009;118(6):1181–1192. doi: 10.1007/s00122-009-0972-y. [DOI] [PubMed] [Google Scholar]
- Mandel J, Dechaine J, Marek L, Burke J. Genetic diversity and population structure in cultivated sunflower and a comparison to its wild progenitor, Helianthus annuus L. Theor Appl Genet. 2011;123(5):693–704. doi: 10.1007/s00122-011-1619-3. [DOI] [PubMed] [Google Scholar]
- Miller J, Hammond J, Roath W. Comparison of inbred vs. single-cross testers and estimation of genetic effects in sunflower. Crop Sci. 1980;20(6):703–706. doi: 10.2135/cropsci1980.0011183X002000060007x. [DOI] [Google Scholar]
- Nambeesan SU, Mandel JR, Bowers JE, Marek LF, Ebert D, Corbi J, Rieseberg LH, Knapp SJ, Burke JM. Association mapping in sunflower (helianthus annuus l.) reveals independent control of apical vs. basal branching. BMC Plant Biol. 2015;15(1):1. doi: 10.1186/s12870-015-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D, Varona L, Ngambi J, Visser D, Mbajiorgu CA, Voordewind S. Estimation of the additive and dominance variances in sa duroc pigs. Livest Sci. 2010;131(1):144–147. doi: 10.1016/j.livsci.2010.03.005. [DOI] [Google Scholar]
- Reif JC, Hahn V, Melchinger AE. Genetic basis of heterosis and prediction of hybrid performance. Helia. 2012;35(57):1–8. doi: 10.2298/HEL1257001R. [DOI] [Google Scholar]
- Rincent R (2014) Optimization of association genetics and genomic selection strategies for populations of different diversity levels: Application in maize (Zea mays L.). Ph.D. thesis, AgroParisTech
- Roath W, Hammond J, Miller J (1982) Genetic effects of days to flowering in sunflower (Helianthus annuus L.) under short day regime. In: Proceedings of the International Sunflower Conference (USA)
- Segura V, Vilhjálmsson BJ, Platt A, Korte A, Seren Ü, Long Q, Nordborg M. An efficient multi-locus mixed-model approach for genome-wide association studies in structured populations. Nat Genet. 2012;44(7):825–830. doi: 10.1038/ng.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandén I, Christensen OF. Allele coding in genomic evaluation. Genet Sel Evol. 2011;43(1):1. doi: 10.1186/1297-9686-43-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G, Christensen OF, Ostersen T, Henryon M, Lund MS. Estimating additive and non-additive genetic variances and predicting genetic merits using genome-wide dense single nucleotide polymorphism markers. PLoS One. 2012;7(9):e45,293. doi: 10.1371/journal.pone.0045293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry JM, Goodman MM, Doebley J, Kresovich S, Nielsen D, Buckler ES. Dwarf8 polymorphisms associate with variation in flowering time. Nat Genet. 2001;28(3):286–289. doi: 10.1038/90135. [DOI] [PubMed] [Google Scholar]
- Tsepilov YA, Shin SY, Soranzo N, Spector TD, Prehn C, Adamski J, Kastenmüller G, Wang-Sattler R, Strauch K, Gieger C, et al. Nonadditive effects of genes in human metabolomics. Genetics. 2015;200(3):707–718. doi: 10.1534/genetics.115.175760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N (2012) Improving crop resistance to abiotic stress, vol. 1. Wiley
- VanRaden P. Efficient methods to compute genomic predictions. J Dairy Sci. 2008;91(11):4414–4423. doi: 10.3168/jds.2007-0980. [DOI] [PubMed] [Google Scholar]
- Waldmann P, Mészáros G, Gredler B, Fuerst C, Sölkner J. Evaluation of the lasso and the elastic net in genome-wide association studies. Front Genet. 2013;4:270. doi: 10.3389/fgene.2013.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Eskridge KM, Crossa J. Identifying qtls and epistasis in structured plant populations using adaptive mixed lasso. J Agri Biol Environ Stat. 2011;16(2):170–184. doi: 10.1007/s13253-010-0046-2. [DOI] [Google Scholar]
- Wang SB, Feng JY, Ren WL, Huang B, Zhou L, Wen YJ, Zhang J, Dunwell JM, Xu S, Zhang YM. Improving power and accuracy of genome-wide association studies via a multi-locus mixed linear model methodology. Sci Rep. 2016;6:19444. doi: 10.1038/srep19444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. Heterosis and the genetics of complex characters. Nature. 1959;184(4685):527–530. doi: 10.1038/184527a0. [DOI] [PubMed] [Google Scholar]
- Wilson RN, Somerville CR. Phenotypic suppression of the gibberellin-insensitive mutant (gai) of arabidopsis. Plant Physiol. 1995;108(2):495–502. doi: 10.1104/pp.108.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li L, Jiang H, Nettleton D, Schnable PS (2014) Dominant gene action accounts for much of the missing heritability in a gwas and provides insight into heterosis. Genome-wide association studies to dissect the genetic architecture of yield-related traits in maize and the genetic basis of heterosis 1001:44
- Yi N, Xu S. Bayesian lasso for quantitative trait loci mapping. Genetics. 2008;179(2):1045–1055. doi: 10.1534/genetics.107.085589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Pressoir G, Briggs WH, Bi IV, Yamasaki M, Doebley JF, McMullen MD, Gaut BS, Nielsen DM, Holland JB, et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet. 2006;38(2):203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ersoz E, Lai CQ, Todhunter RJ, Tiwari HK, Gore MA, Bradbury PJ, Yu J, Arnett DK, Ordovas JM, et al. Mixed linear model approach adapted for genome-wide association studies. Nat Genet. 2010;42(4):355–360. doi: 10.1038/ng.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Chen Y, Yao W, Zhang C, Xie W, Hua J, Xing Y, Xiao J, Zhang Q. Genetic composition of yield heterosis in an elite rice hybrid. Proceed Natl Acad Sci. 2012;109(39):15,847–15,852. doi: 10.1073/pnas.1214141109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat Genet. 2012;44(7):821–824. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.