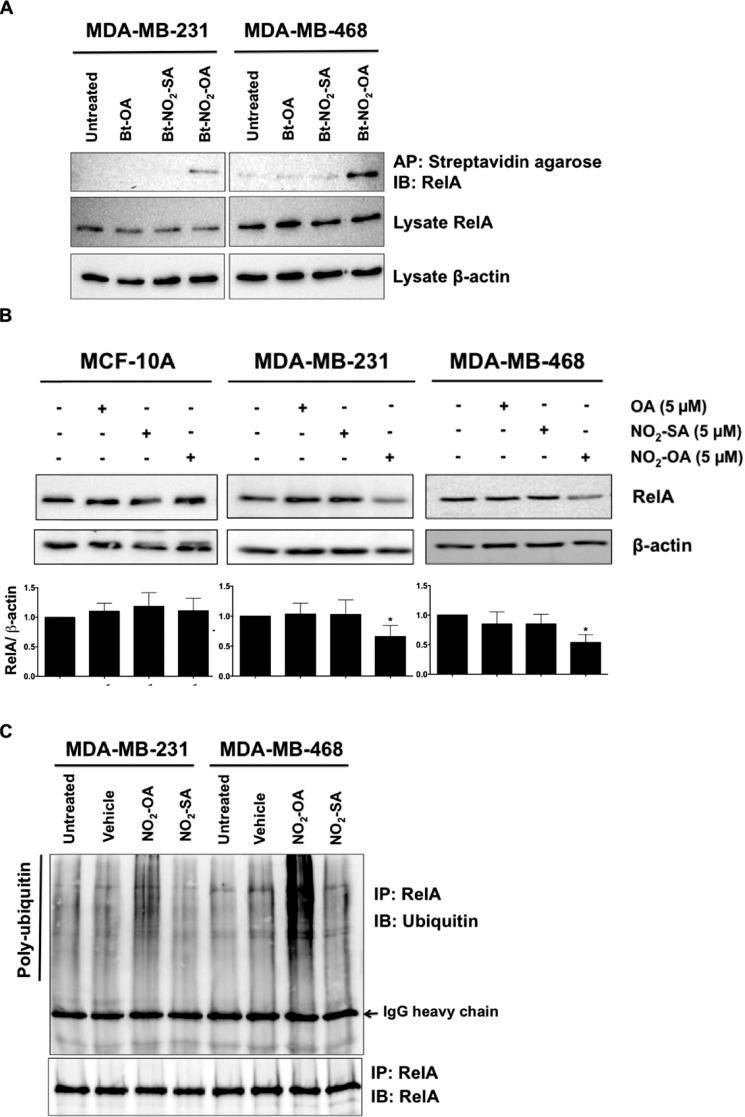
Figure 8.
NO2-OA alkylates and destabilizes NF-κB RelA protein in TNBC cells. A, MDA-MB-231 or MDA-MB-468 cells were treated with 5 μm Bt-NO2-OA, Bt-NO2-SA, or Bt-OA for 2 h. After cell lysis, biotinylated NO2-FAs with adducts were affinity-purified (AP) using streptavidin-agarose beads. Pulled-down RelA protein was then detected by immunoblotting (IB). RelA and control β-actin immunoblots from the same input lysates used for affinity purification are shown below the panel. B, endogenous RelA protein levels were detected by immunoblotting probed with anti-RelA antibody using β-actin as a loading control. The relative total RelA levels (normalized by total β-actin) compared with untreated controls were quantified. *, p < 0.05 versus untreated control. Significance was determined by one-way analysis of variance followed by Tukey post test. C, MDA-MB-231 or MDA-MB-468 cells were treated with vehicle (methanol), NO2-OA (5 μm), or NO2-SA (5 μm) for 6 h, and then cell lysates were harvested and immunoprecipitated (IP) by anti-RelA antibody followed by immunoblotting. Pulldown level of immunoprecipitated RelA proteins is shown below the panel.