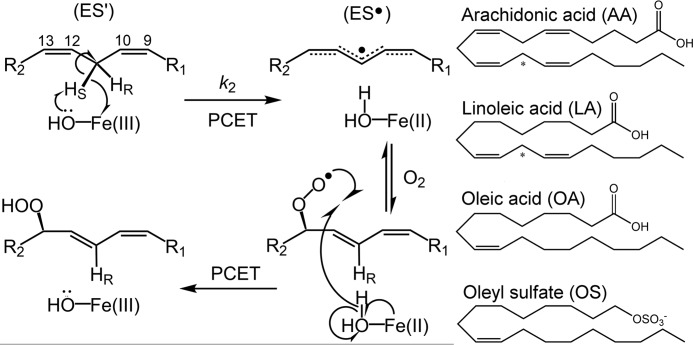
Figure 2.
Mechanism of lipid peroxidation by lipoxygenase. Left, reaction of model enzyme, soybean lipoxygenase, with substrate linoleic acid as an example. The metallocofactor, often a ferric hydroxide, although a manganese center is employed by fungal enzymes (63), abstracts a hydrogen atom from polyunsaturated fatty acids, in a regio- and stereospecific manner, through a proton-coupled electron transfer (PCET) mechanism (49). Molecular oxygen inserts into the delocalized radical intermediates, forming the product upon reverse proton-coupled electron transfer. The carbon numbering of the LA substrate is shown for reference. Relevant microscopic rate constant, k2, associated with the rate-determining C–H abstraction, is labeled. The enzyme-substrate and enzyme-LA radical complexes are labeled as ES′ and ES•, respectively. Right, the structures of substrates, as well as the allosteric effectors OA and OS, are shown for reference. The reactive carbon for each substrate is designated by an asterisk.