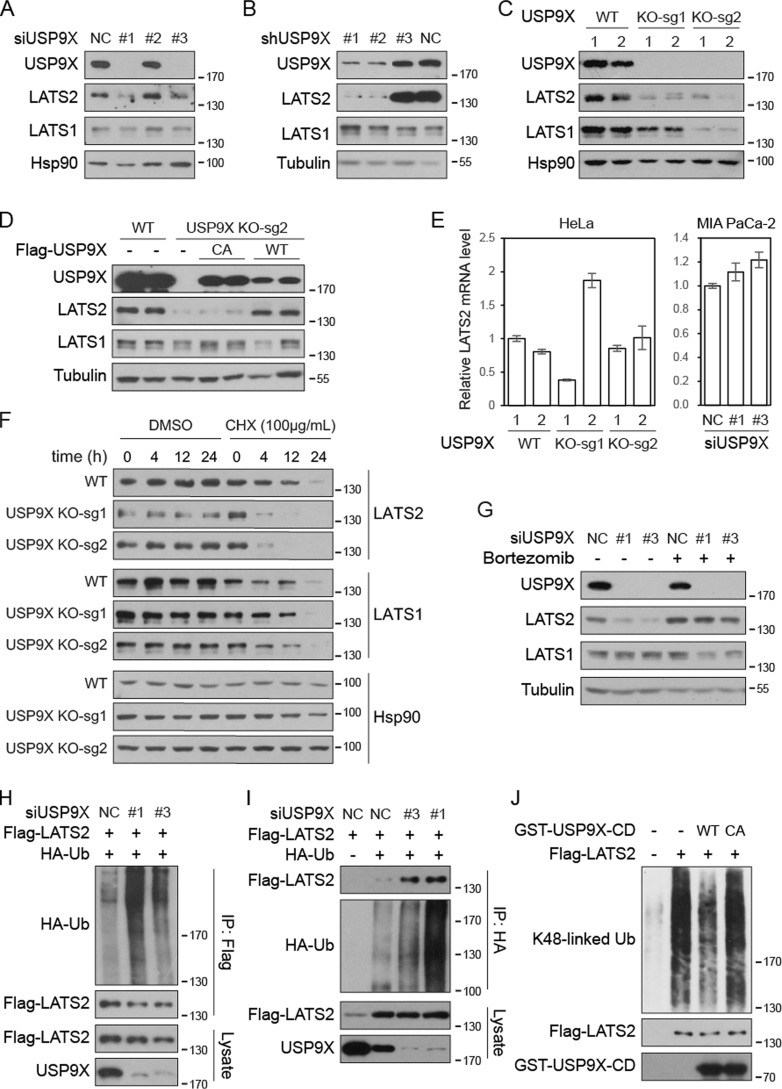
Figure 4.
USP9X deubiquitinates and stabilizes LATS2. A and B, knockdown of USP9X diminishes LATS2 protein level. USP9X was knocked down by siRNAs in MIA PaCa-2 cells (A) or by shRNAs in BxPC-3 cells (B). Cell lysates were examined by Western blotting. C, knockout of USP9X diminishes LATS2 protein level. USP9X was knocked out in HeLa cells by CRISPR-Cas9 technology using two independent sgRNAs. Two clones were examined for each genotype. D, enzymatically inactive USP9X fails to rescue LATS2 protein level in USP9X-knockout cells. FLAG-USP9X-WT and CA mutant were expressed in USP9X-knockout MIA PaCa-2 cells. Cell lysates were examined by Western blotting. E, loss of USP9X does not inhibit LATS2 mRNA expression. The mRNA levels of LATS2 in USP9X-knockout HeLa cells or USP9X knockdown MIA PaCa-2 cells were determined by quantitative RT-PCR. Values represent means ± S.D. (error bars) from three technical repeats. F, LATS2 is destabilized in USP9X-knockout cells. Wild-type and USP9X-knockout HeLa cells were treated with cycloheximide (CHX) as indicated before collection. Cell lysates were examined by Western blotting. G, proteasome inhibitor rescues LATS2 protein level in USP9X knockdown cells. Wild-type and USP9X knockdown MIA PaCa-2 cells were treated with 100 nm bortezomib for 24 h before collection. H and I, knockdown of USP9X promotes LATS2 ubiquitination. LATS2 and siRNAs were transfected into HeLa cells stably expressing HA-Ub or control HeLa cells, as indicated. FLAG-LATS2 (H) or HA-Ub (I) was immunoprecipitated (IP) with anti-FLAG or anti-HA antibodies, respectively. Samples were examined by Western blotting. J, USP9X deubiquitinates LATS2 in vitro. Wild-type or inactive catalytic domain of USP9X was purified from E. coli. FLAG-LATS2 was immunoprecipitated from bortezomib-treated transfected HeLa-Ub stable cells. LATS2 and USP9X catalytic domain were then incubated in deubiquitination assay buffer. Samples were analyzed by Western blotting. Data are representative of triplicate experiments.