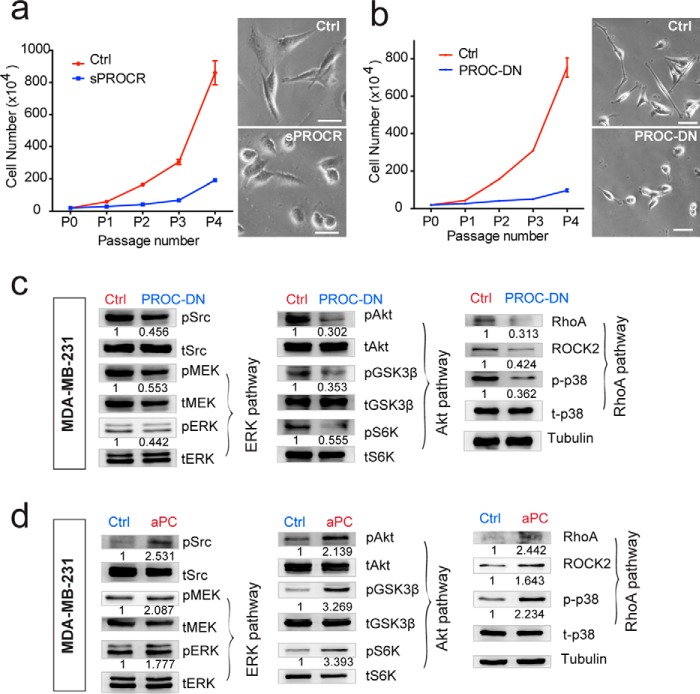
Figure 7.
Protein C serves as the ligand for the activation of PROCR intracellular signaling in breast cancer cells. a, MDA-MB-231 cells were cultured in the presence of Ctrl or sPROCR (6 μg/ml) for four passages in complete media. Cell numbers that are counted in each passage showing that sPROCR markedly inhibited cell proliferation. The spindle-shaped morphology of MDA-MB-231 (Ctrl, upper right panel) was altered to become more spherical in the presence of sPROCR (bottom right panel). One of three similar experiments is shown. Data are presented as mean ± S.E. b, MDA-MB-231 cells were cultured in the presence of Ctrl or PROC-DN (2 μg/ml) for four passages in complete media. Cell numbers that are counted in each passage show that PROC-DN markedly inhibited cell proliferation. The spindle-shaped morphology of MDA-MB-231 (Ctrl, upper right panel) was altered to become more spherical in the presence of PROC-DN (bottom right panel). One of three similar experiments is shown. Data are presented as mean ± S.E. c, Western blot showing that addition of PROC-DN in MDA-MB-231 cells (24 h) diminishes the activities of Src and all three PROCR intracellular signaling pathways (ERK, PI3K–Akt–mTOR, and RhoA–ROCK signaling). d, Western blot showing that addition of active PROC (10 μg/ml) in MDA-MB-231 cells (8 h) enhances the activities of Src and all three PROCR intracellular signaling pathways. Western blots in the same panel are from the same batch of cells using the same loadings, thus only one loading control is shown at the end of the panel. For a better illustration, they are shown as three separated columns representing ERK, Akt, and RhoA pathways, respectively. Each experiments was repeated three times or more.