Key Points
Success of hematopoietic stem cell transplantation for MIRAGE syndrome may be limited by syndrome-specific comorbidities.
SAMD9 mutations associated with MIRAGE syndrome are a newly described cause of congenital amegakaryocytic thrombocytopenia.
Introduction
MIRAGE syndrome is a recently described disorder consisting of myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital abnormalities, and enteropathy. It was initially identified in a subset of Japanese patients with syndromic adrenal hypoplasia and is caused by mutations in the SAMD9 gene on chromosome 7, which encodes a protein involved in endosome trafficking.1,2 Because endosome function is essential for a diverse array of cellular and organ-specific processes, the phenotypes associated with mutations in SAMD9 are quite varied.1-5 The majority of patients die in infancy because of infections or adrenal crises, whereas other patients with monosomy 7 at diagnosis developed myelodysplastic syndromes (MDS) and died of related complications.2 Interestingly, most MIRAGE patients reported to date had at least transient anemia and thrombocytopenia, although several had refractory anemias or MDS (Table 1).1,2
Table 1.
Clinical characteristics of 6 patients with MIRAGE syndrome who have undergone HSCT
| Patient 1 | Patient 2 | Patient 31 | Patient 41 | Patient 52 | Patient 66 | |
|---|---|---|---|---|---|---|
| Status | Deceased | Deceased | Alive | Alive | Deceased | Alive |
| Constitutional karyotype | 46,XX | 46,XY | 46,XY | 46,XY | 46,XX | 46,XY |
| SAMD9 mutation | c.2462A>T; p.K821M | c.2920G>A; p.E974K | c.4707G>T; p.K1569N | c.2948T>G; p.I983S | c.2305G>A; p.D769N | c.2691A>G; p.I897M |
| Ethnicity | White | White/Asian | NA | NA | Asian | White |
| Age at delivery, wk | 28 | 30 | 37 | 36 | 32 | 32 |
| IUGR | Present | Present | Present | Present | Present | Present |
| Adrenal insufficiency | Severe | Severe | Mild | None | Severe | Severe |
| Genitourinary malformations | Vesicoureteral reflux and pelviectasis | Microphallus | Male w/ hypospadias | Female with slight clitoromegaly | Absent | Microphallus, hypospadias, cryptorchidism |
| Gonads | NE | Undescended testes bilaterally | Descended testes | Inguinal testes | NR | Testicular failure |
| Blood dyscrasias | ||||||
| Thrombocytopenia | Severe at birth | Developed within 2 mo | Mild | Severe | Mild (no transfusions) | NR |
| Anemia | Severe, required transfusions | Developed within 2 mo | Absent | Absent | Absent | NR |
| Other hematological disorder | CAMT | Monosomy 7 MDS | Monosomy 7 MDS | Monosomy 7 MDS | Monosomy 7 MDS/AML | Monosomy 7 MDS |
| Sepsis | Yes | Yes | Yes | Yes | None | Yes |
| Feeding intolerance | Present since birth, NG dependent on elemental formula | Present since birth, NG dependent on elemental formula | NR | NR | None | Uncoordinated swallowing and esophageal stricture |
| Chronic diarrhea | Present | Present | Present | Present | Present | NR |
| Bronchopulmonary dysplasia | Present | Present, hypoxic with mild illnesses | NR | NR | None | Bronchiectasia |
| Age at HSCT | 14 mo | 16 mo | NR | NR | 4 y | 6 y |
| HSCT type | RI, flu+thio+mel+ATG, MURD | RI, flu+TBI, MRD | MURD | MURD | MRD | RI, flu+mel, MURD |
| GVHD prophylaxis | CSA, MMF | Tac, MMF | NR | NR | NR | CSA, Pred, MTX |
| Complications | Infections, cytopenias, enteropathy, adrenal crises | Infections, cytopenias, enteropathy, adrenal crises | NR | NR | EBV-PTLD, respiratory failure | Respiratory infections, skin GVHD |
| Age at death | 23 mo | 21 mo | NA | NA | 5 y | NA |
Comparison of patients with SAMD9-related hematological disorders associated with MIRAGE syndrome who underwent HSCT. Patients 1 and 2 are from this report. Patients 3 to 6 were published elsewhere.
AML, acute myeloid leukemia; ATG, antithymocyte globulin; CAMT, congenital amegakaryocytic thrombocytopenia, CSA, cyclosporine; EBV, Epstein-Barr virus; flu, fludarabine; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; IUGR, intrauterine growth restriction; mel, melphalan; MMF, mycophenolate motil; MTX, methotrexate; MURD, matched unrelated; NA, not applicable; NE, not evaluated; NG, nasogastric feeds; NR, not reported; pred, prednisone; PTLD, posttransplant lymphoproliferative disorder; RI, reduced intensity; tac, tacrolimus; TBI, total body irradiation; thio, thiotepa.
Because the SAMD9 gene resides on the long arm of chromosome 7 and MIRAGE-associated mutations likely function through gain of function, affected individuals can develop adaptive clones that have lost the disease-causing allele carried on chromosome 7q.1,2 Although loss of chromosome 7 or its long arm is presumably adaptive and hypothesized to allow for more rapid cellular growth, these clones are also associated with MDS.1,2 The appropriate management of patients with MIRAGE syndrome and associated non–leukemic hematological disorders is not currently known. To this end, we report 2 cases of HSCT in patients with hematological disorders associated with MIRAGE syndrome, one of which is the first case of congenital amegakaryocytic thrombocytopenia associated with MIRAGE. We also highlight the severe multiorgan complications intrinsic to patients with SAMD9 mutation–positive MIRAGE syndrome that complicate allogeneic HSCT.1
Case description
Patient 1 was a female born at 28 weeks’ gestation due to severe IUGR. In addition to multiple complications that were initially attributed to her prematurity, she experienced several unusual complications, including electrolyte imbalances due to what was thought at the time to be pseudohypoaldosteronism and vesicoureteral reflux with bilateral renal caliectasis. In addition, she had severe thrombocytopenia requiring platelet transfusions from birth on a weekly basis to maintain platelet count >10 × 109/L. Serum thrombopoietin level was high at 3832 pg/mL (normal range 7-99 pg/mL). A bone marrow aspirate performed at 4 months of age revealed a normocellular marrow, with normal myeloid and erythroid differentiation (M:E ratio 3.5:1) but absent megakaryocytes, suggesting a diagnosis of congenital amegakaryocytic thrombocytopenia. Direct sequencing studies revealed no mutations, deletions, or duplication in MPL, or in other genes associated with inherited thrombocytopenias and marrow failure (eg, RUNX1, WAS, MYH9, SBDS). Whole exome sequencing performed at 5 months was initially reported as finding no known pathogenic variants. Over the next several months, the patient continued to have slowly progressive bone marrow failure, with increasing platelet and red blood cell transfusion requirements, as well as onset of neutropenia. Her course was further complicated by 4 episodes of bacterial line infections (Enterococcus, Klebsiella) with clinical features of sepsis. A repeat bone marrow evaluation was performed at 13 months of age that demonstrated profound hypocellularity (5% to 10%) with absent megakaryocytes, but without dysplasia (Figure 1A). Cytogenetic evaluation from this bone marrow revealed no rearrangements or copy number abnormalities by either G-banding metaphase karyotype analysis or whole genome single-nucleotide polymorphism array. Single-nucleotide polymorphism array specifically did not identify copy neutral loss of heterozygosity or deletion of any portions of chromosome 7.
Figure 1.
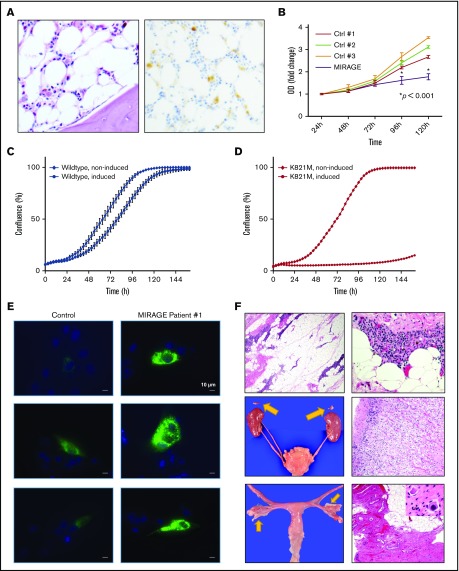
Confirmation of MIRAGE syndrome phenotype in Patient 1. (A) PAS (left; 50× magnification) and CD42 (right; 100× magnification) stain of bone marrow biopsy from patient 1 pre–stem cell transplantation , demonstrating hypocellularity and complete absence of megakaryocytes, with hemosiderin deposits representing the only source of stain on the CD42-stained image (right). (B) Cell proliferation assay measuring growth of primary skin fibroblasts from MIRAGE syndrome patient 1 (blue) vs control patients from the Children's Hospital of Philadelphia bone marrow failure registry (other colors) demonstrated poor growth in fibroblasts with the SAMD9 mutation causing MIRAGE (representative of 3 independent assays). (C-D) Modeling of the K821M substitution shows potent growth inhibition in vitro. Cell proliferation of stably transfected HEK293 cells that overexpress each SAMD9 protein (wild type or K821M) were evaluated with a time-lapse microscope. SAMD9 expression was induced by adding Doxycycline (1 μg/mL) in the culture media. (C) Induced expression of the wild-type protein caused mild growth suppression. (D) Induced expression of the K821M mutant caused profound growth suppression. Data are means of triplicate experiment. Bars indicate standard error. (E) Representative fluorescence micrographs of fibroblasts transfected with a late endosomal marker construct (GFP-RAB7A) from a control subject (left) and MIRAGE patient 1 (right), taken at 63× magnification with identical exposure parameters, demonstrating increased large GFP-positive RAB7A vesicles in fibroblasts from MIRAGE patient 1. (F) Hematoxylin and eosin–stained micrographs from patient 1 demonstrating thymic hypoplasia with lymphocyte depletion, lack of corticomedullary demarcation, absence of Hassl corpuscles, and extensive adipocytic infiltration (top panels; left 40× and right 400× magnification). The thymus weighed 1.13 g (normal for height: 14.5 g).7 Gross (arrows, middle left) and microscopy (middle right; 200× magnification) of bilateral adrenal hypoplasia with preserved cortical zonation. Combined weight = 0.24 g (normal for height: 5.9 g).6 Bilateral ureteral duplication (middle left). Gross image of bilateral atrophic ovaries (bottom left, arrows) and microscopic features of atrophic ovaries (bottom right; 40× magnification) with fibrous stroma and paucity of germ cells. Very rare primordial follicles were identified (inset; 600× magnification). OD, optical density.
Because of worsening bone marrow failure, she was evaluated for HSCT. Because she did not have siblings, a 9/10 HLA-matched donor was identified (single-antigen mismatch at HLA-C, although fully matched also at HLA-DPB1), and at 14 months of life, she underwent an unrelated donor peripheral blood stem cell transplantation with reduced intensity conditioning consisting of thymoglobulin (9 mg/kg total dose), fludarabine (200 mg/m2), thiotepa (10 mg/kg), and melphalan (140 mg/m2). To minimize risks of potential organ toxicity and GVHD, partial ex vivo T-cell depletion was performed per an expanded access protocol for CD3+/CD19+ cell depletion (NCT02356653). GVHD prophylaxis consisted of mycophenolate mofetil and cyclosporine. The patient tolerated conditioning well and received a CD3+/CD19+-depleted graft consisting of 8.06 × 106 CD34+ cells per kilogram, with an add back of 1 × 105 CD3+ T cells per kilogram. Neutrophil engraftment (defined by the first of 3 absolute neutrophil count values exceeding 500 cells per microliter) was achieved by day +10, and definitive platelet engraftment (platelet count >50 000 platelets per microliter a week after last platelet transfusion) was achieved by day +32. Initial and all subsequent donor chimerism evaluations revealed 100% donor chimerism in all blood cell lineages.
Despite good engraftment, the patient’s posttransplant course was striking because of a plethora of unusual complications, which included enteral feeding intolerance with intermittent nonbloody voluminous diarrhea resembling dumping syndrome, but without convincing biopsy evidence of GVHD; severe renal insufficiency exacerbated by even low-dose calcineurin inhibitor therapy; severe electrolyte imbalances consisting primarily of hypernatremia and hypokalemia requiring IV fluid and electrolyte supplementation; frequent (several times per week) episodes of adrenal crisis and/or dysautonomia in which she would become febrile, hypotensive, and hypoxic, with poor peripheral perfusion, typically requiring pressor support and ultimately leading to chronic steroid dependence; and 10 distinct episodes of culture-positive bacteremia, all occurring after initial engraftment, with organisms including Klebsiella pneumoniae, Bacillus species, Staphylococcus haemolyticus, Pseudomonas aeruginosa, and Enterococcus faecalis. Her other complications, occurring at ∼4 months after transplant, included progressive cytopenias with an immune-mediated component, most striking of which was agranulocytosis associated with a monoclonal donor-derived T-cell population in marrow that developed at ∼5 months posttransplant and eventually responded to lymphocyte-directed combination therapy with cyclophosphamide, alemutuzumab, and vincristine. Unfortunately, because of multiple complications, she developed chronic ventilator-dependent respiratory failure from which she was unable to recover. She eventually was transitioned to comfort measures and died at 9 months posttransplant.
Approximately 3 months after patient 1 had undergone HSCT, the initial study by Narumi et al describing MIRAGE syndrome was published.2 At this time, our patient’s whole exome sequencing was requeried, and she was discovered to have a de novo heterozygous missense variant (c.2462A>T; p.K821M) in exon 3 of SAMD9 at a variant allele frequency consistent with a germline heterozygous mutation. In silico analysis using PolyPhen-2 predicted that the resulting amino acid substitution was likely deleterious to protein function. Neither parent possessed this variant. In addition, this variant had not been observed in the exome aggregation consortium database. In accordance with our institutional Internal Review Board, and with the informed consent from her parents, she was enrolled to participate in our institutional Bone Marrow Failure Registry and Sample Repository. To verify the pathogenicity of the patient’s SAMD9 variant in vitro, we obtained the patient’s skin biopsy fibroblasts (see supplemental Methods) and found that, similar to the original MIRAGE syndrome description,1,2 primary skin fibroblasts from our patient grew significantly more slowly in culture compared with control fibroblasts from our repository (Figure 1B). To further investigate the impact of the c.2462A>T mutation on SAMD9 function, we performed growth experiments using cell lines stably transfected with either wild-type SAMD9 or with SAMD9 containing the (c.2462A>T; p.K821M) mutation. Induction of SAMD9 transgene expression with doxycycline showed marked growth inhibition compared with wild-type SAMD9, with negligible growth at 144 hours in cells expressing the mutant SAMD9 construct (Figure 1C-D). This result clearly demonstrates that the mutation identified in this patient leads to potent growth inhibition in vitro and likely in vivo as well.
In addition, when transfected with a construct expressing a labeled late endosome marker (GFP-RAB7a), the cytoplasm of primary fibroblasts from our patient demonstrated an expansion of GFP-bright, RAB7a-positive vesicles compared with control fibroblasts, consistent with the cellular phenotype previously seen in fibroblasts derived from MIRAGE syndrome patients (Figure 1E). Finally, autopsy analysis demonstrated several profound anatomic abnormalities previously described in patients with MIRAGE syndrome,2 including thymic dysplasia characterized by absent Hassl corpuscles and lack of corticomedullary differentiation, bilateral atrophic ovaries, and profound adrenal hypoplasia (Figure 1F).
Patient 2 was a male born at 30 weeks 5 days’ gestation by cesarean section due to severe IUGR (birth weight: 980 g). He had congenital adrenal hypoplasia, hypothyroidism, severe growth restriction, micropenis, chronic diarrhea with duodenitis and colitis indicative of an enteropathy, recurrent infections, pulmonary hypoplasia, and temperature instability with anhidrosis. He required prolonged intensive care for 131 days because of feeding intolerance, recurrent infections, and chronic lung disease of prematurity. The patient received donor breast milk for the first 2 months of life and was subsequently discharged on gastrostomy tube feeding after being transitioned to an elemental formula. During the first year of life, the patient had multiple pediatric intensive care unit admissions for infections primarily of viral origin, including rhinovirus and coronavirus, which were complicated by shock secondary to adrenal insufficiency.
Given the history of recurrent infections in the setting of low immunoglobulin G levels requiring intravenous immunoglobulin replacement and a persistent normocytic nonhemolytic anemia, a bone marrow biopsy was performed at 1 year of age. An MDS fluorescence in situ hybridization (FISH) panel revealed monosomy 7 in 16% of cells with an additional 68% of cells having a deletion of 7q. Giemsa stain of metaphase spreads showed a male karyotype with loss of 1 copy of chromosome 7 in 95% of mitoses analysed: 45,XY, −7[19]/XY[1]. The discrepancy between the MDS FISH panel and metaphase spread analysis may be related to a growth advantage conferred to the monosomy 7 cells. Trilineage hematopoiesis with dyserythropoiesis and diminished numbers of megakaryocytes, consistent with a diagnosis of refractory cytopenia of childhood, was observed as well. Skin fibroblast testing did not demonstrate any constitutional cytogenetic abnormalities. Repeat bone marrow biopsy 2 months later confirmed persistent dyserythropoiesis and megakaryocyte hypoplasia with monosomy 7 in 90% of cells by FISH. Telomere length analysis, chromosome breakage studies, and a bone marrow failure syndrome panel including MPL, RUNX1, WAS, MYH9, DKC1, TERC, TERT, TINF2, and SBDS were negative. The patient’s clinical features of myelodysplasia, adrenal insufficiency, genitourinary anomalies, and enteropathy raised concern for MIRAGE syndrome; therefore, SAMD9 sequencing was performed and identified a missense c.2920G>A; p.E974K mutation that was previously reported in a patient with MIRAGE syndrome.2 Because of concern for progression of the patient’s MDS, the patient underwent a matched related donor (MRD) HSCT in accordance with the center’s regulatory standards and a center-specific protocol for nonmyeloablative conditioning with fludarabine (90 mg/m2) and total body irradiation (300 cGy). He tolerated the conditioning regimen and stem cell infusion (49.52 × 106 CD34+ cells) without complications. Posttransplant GVHD prophylaxis included mycophenolate mofetil and tacrolimus, which was also tolerated without major complications. The patient engrafted on day +16, at which time he started to demonstrate recurrent episodes of temperature instability, with core temperature dropping as low as 35°C. He received stress dose hydrocortisone, and sepsis evaluations were undertaken, which did not identify any organisms. The patient continued to experience frequent episodes of temperature instability, with temperatures ranging between 35°C and 39°C without any other associated symptoms other than tachycardia with fever. The patient also lost the ability to tolerate feeds because of emesis and diarrhea, and the decision was made to discontinue enteral feeding and provide total parenteral nutrition. Endoscopy showed only mild gut GVHD. Multiple chest computed tomography scans due to hypoxia and fever showed only parenchymal disease without focal consolidations or nodules. The etiology of the parenchymal disease was believed to be related to pulmonary hypoplasia. On day +110, the patient had a pulseless electrical activity arrest with acute respiratory distress syndrome secondary to K pneumoniae sepsis requiring extracorporeal membrane oxygenation and continuous renal replacement therapy due to acute kidney injury. Unfortunately, the patient subsequently developed necrotizing fasciitis requiring emergent debridement and died due to cardiopulmonary arrest within a month of coming off of extracorporeal membrane oxygenation.
Discussion
MIRAGE syndrome is a recently described form of syndromic congenital adrenal insufficiency, caused by heterozygous germline missense mutations in SAMD9 with clinical features that include MDS, infection, restricted growth, adrenal hypoplasia, ambiguous genitalia, and enteropathy.2 In this report, we describe the first patient to present with congenital amegakaryocytic thrombocytopenia secondary to MIRAGE syndrome and describe a novel disease-causing SAMD9 allele (c.2462A>T; p.K821M). In addition, we also describe another patient with MDS secondary to MIRAGE syndrome and highlight the experiences of HSCT for patients with this condition. In both cases, the HSCT course was complicated by features of the underlying genetic syndrome and resulted in death of both of the patients shortly following HSCT despite engraftment. We also contrast the patients reported here with 4 previously reported patients who have undergone HSCT for SAMD9-related bone marrow failure (Table 1), providing the largest compilation of patients who have undergone HSCT for SAMD9-associated marrow failure.
Several of the pretransplant comorbidities noted in both patients described above, including temperature instability, enteropathy, adrenal crises, predisposition to bacteremia, electrolyte imbalances, and preexisting lung disease, significantly complicated the posttransplant course. The innate temperature and blood pressure instability were particularly vexing in both cases, because the patients required empiric antimicrobial therapy for the entirety of the posttransplant course and spent prolonged periods of time in the intensive care unit. In addition, neither patient was able to fully tolerate enteral feeds likely because of worsening of the MIRAGE-associated enteropathy without evidence of significant lower-tract GVHD. Most alarmingly, in contrast to other types of immune deficiencies or bone marrow failure where HSCT is used as a definitive treatment strategy to decrease infection risk and improve long-term survival, in these 2 patients with severe MIRAGE syndrome, further deterioration in immune and epithelial function caused by HSCT and regimen-related toxicities led to increasing frequency and severity of infections, including necrotizing fasciitis in patient 2, which ultimately led to their deaths.
The similarities in complications reported for the 2 MIRAGE patients with distinct hematological disorders suggest that regardless of the diagnosis of bone marrow failure or monosomy 7 MDS, patients with severe MIRAGE syndrome phenotypes may not be amenable to HSCT (Table 1). Two recent reports described successful HSCT outcomes for patients with germline SAMD9 mutations, 2 of whom did not have MIRAGE syndrome and 2 others with mild phenotypes.1,4 Critically, these patients had many fewer pre-HSCT comorbidities and did not have severe adrenal insufficiency or enteropathy (Table 1).1,4 In fact, all 3 patients with MIRAGE and severe adrenal insufficiency who were transplanted in infancy, including the 2 cases presented here, died of posttransplant complications (Table 1), whereas 2 patients in Table 1 and 2 reported elsewhere4 who had no or only mild adrenal insufficiency underwent successful HSCT for SAMD9-related MDS. Another patient with MIRAGE syndrome who presented later in childhood with neutropenia that transitioned to MDS underwent successful HSCT at age 5.6 Although he continues to have numerous disease-related comorbidities, he is currently alive.6 Despite the patient having an immunodeficiency and adrenal insufficiency, the severe chronic diarrhea associated with MIRAGE syndrome was not present, and he was transplanted at an older age, suggesting a less severe MIRAGE phenotype.6
Although it was anticipated that HSCT would treat the associated hematological disorders and improve infectious complications in patients with severe MIRAGE phenotypes, this was not the outcome for either patient reported here. Reasons for continued infections in these patients include need for multiple central lines, posttransplant immunosuppression, and underlying epithelial barrier dysfunction that may have been exacerbated by conditioning regimens. In addition, similar to concerns in patients with DiGeorge syndrome, the profound thymic dysplasia seen in this patient population may result in impaired normal polyclonal T-cell reconstitution following stem cell transplantation, predisposing to T-cell dysregulation consequences such as autoimmune cytopenias, and/or contributing to ongoing infection risk. Neither patient’s quality of life was improved by transplant, nor did HSCT clearly extend life expectancy in these patients despite successful engraftment. Therefore, careful consideration of the benefits and risks of HSCT in patients with severe MIRAGE syndrome phenotypes is warranted before proceeding with HSCT, even with reduced-intensity conditioning regimens, and families should be aware of the potential for a protracted and particularly arduous post-HSCT course. In addition, new specialized approaches for management of multiorgan complications of MIRAGE syndrome are needed, particularly for patients with underlying adrenal insufficiency.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are grateful to Leslie Kean for helpful comments with the second patient and discussion. The authors acknowledge the parents and families of both patients discussed herein for their tireless care and advocacy for their children, and for support of these studies and this manuscript.
This work was supported by National Institutes of Health (NIH), National Cancer Institute grant T32 CA009351 (J.S.), NIH, National Heart, Lung, and Blood Institute grant K08 HL122306, and NIH, National Institute of Diabetes and Digestive and Kidney Diseases grant R24 DK103001 (T.S.O.).
Authorship
Contribution: J.Z., D.B., and J.-M.F. developed and performed the skin fibroblast studies; A.S. contributed autopsy images and interpretation; G.W. contributed bone marrow sections and interpretation; A.H. and A.M. contributed to the case report; H.S. and S.N. performed growth experiments; S.N., J.S., and T.S.O. prepared the manuscript; A.T., S.Y., S.M., and D.B. assisted with revisions; and T.S.O. contributed to the case report and oversaw manuscript development.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timothy S. Olson, Children's Hospital of Philadelphia, 3615 Civic Center Blvd, Philadelphia, PA 19104; email: olsont@email.chop.edu.
References
- 1.Buonocore F, Kühnen P, Suntharalingham JP, et al. . Somatic mutations and progressive monosomy modify SAMD9-related phenotypes in humans. J Clin Invest. 2017;127(5):1700-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narumi S, Amano N, Ishii T, et al. . SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet. 2016;48(7):792-797. [DOI] [PubMed] [Google Scholar]
- 3.Chefetz I, Ben Amitai D, Browning S, et al. . Normophosphatemic familial tumoral calcinosis is caused by deleterious mutations in SAMD9, encoding a TNF-alpha responsive protein. J Invest Dermatol. 2008;128(6):1423-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz JR, Wang S, Ma J, et al. . Germline SAMD9 mutation in siblings with monosomy 7 and myelodysplastic syndrome. Leukemia. 2017;31(8):1827-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topaz O, Indelman M, Chefetz I, et al. . A deleterious mutation in SAMD9 causes normophosphatemic familial tumoral calcinosis. Am J Hum Genet. 2006;79(4):759-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson D, Bessler M, Ferkol T, et al. . Comment on: acquired monosomy 7 myelodysplastic syndrome in a child with clinical features of dyskeratosis congenital and IMAGe association [published online ahead of print 17 August 2017]. Pediatr Blood Cancer. doi:10.1002/pbc.26747. [DOI] [PubMed] [Google Scholar]
- 7.Stowens D. Pediatric Pathology. Baltimore, MD: Williams & Wilkins; 1959. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.