Abstract
Androgens act through the nuclear androgen receptor (AR) to regulate gonad differentiation and development. In mice, AR is necessary for spermatogenesis, testis development, and formation of external genitalia in males and oocyte maturation in females. However, the extent to which these phenotypes are conserved in nonmammalian vertebrates is not well understood. Here, we generate zebrafish with a mutation in the ar gene (aruab105/105) and examine the role of AR in sexual determination and gonad development. We found that zebrafish AR regulates male sexual determination, because the majority of aruab105/105 mutant embryos developed ovaries and displayed female secondary sexual characteristics. The small percentage of mutants that developed testes displayed female secondary sexual characteristics, exhibited structurally disorganized testes, and were unable to release or produce normal levels of sperm, demonstrating that AR is necessary for zebrafish testis development and fertility. In females, we found that AR regulates oocyte maturation and fecundity. The aruab105/105 mutant females developed ovaries filled primarily with immature stage I oocytes and few mature stage III oocytes. Two genes whose expression is enriched in wild-type ovaries compared with testes (cyp19a1a, foxl2a) were upregulated in ar mutant testes, and two genes enriched in testes (amh, dmrt1) were upregulated in ar mutant ovaries. These findings demonstrate that AR regulates sexual determination, testis development, and oocyte maturation and suggest that AR regulates sexually dimorphic gene expression. The ar mutant we developed will be useful for modeling human endocrine function in zebrafish.
The AR regulates sex determination and gonad development in zebrafish.
Androgens are hormones necessary for proper sex differentiation and development in vertebrates. Androgens act by binding to receptors, such as the androgen receptor (AR), a ligand-dependent transcription factor and member of the nuclear hormone receptor superfamily (1–7). Studies in mice with mutations in the ar gene demonstrate that in males, AR is required for spermatogenesis and proper development of the internal reproductive tract and external genitalia (8–10). In females, AR is necessary for folliculogenesis, with impaired oocyte maturation observed in AR mutant mice (11). However, the conservation of these phenotypes outside mammals is not well understood and has not been explored in zebrafish, a powerful model for the development and function of the human endocrine system.
Zebrafish exhibit unique sex determination and differentiation mechanisms. Laboratory strains lack a sex chromosome, and no sex-determining gene has been identified (12, 13). Gonadal development of all juvenile zebrafish proceeds via a bipotential gonad that appears histologically as an immature ovary (14). In female zebrafish this bipotential gonad matures into a functional ovary, whereas in males the bipotential gonad regresses and is replaced by a testis (15). Signaling from primordial germ cells (PGCs) is important for female sex differentiation, because ablating PGCs during development produced adult fish that possess a testis but lack sperm (16–19).
Evidence suggests that multiple sex-related genes may be interacting as a network to establish sex, a process described as polygenic sex determination (13). Genes known to play an important role in zebrafish sex determination include doublesex and mab-3 related transcription factor (dmrt1), anti-Müllerian hormone (amh), sry-related HMG box gene 9 (sox9a), forkhead box L2a (foxl2a), and the aromatase enzyme cyp19a1a (17, 20–24). In zebrafish, dmrt1 is necessary for male sex determination and development, with mutant zebrafish lacking functional dmrt1 exhibiting a female sex bias and mutant males displaying testicular dysgenesis (21). Similarly, another study found that dmrt1 was necessary for differentiation of male germ cells, with inhibition of germ cell self-renewal and increased apoptosis occurring in testes of dmrt1 mutants (24). sox9a is expressed in wild-type testes but not in ovaries (20), suggesting that it influences testis differentiation. Cyp19a1a, the gonadal form of aromatase, is upregulated in the ovary compared with the testis in zebrafish (25). Zebrafish embryos with mutations in cyp19a1a develop into males, not females (26).
Evidence suggests that a growth factor released by PGCs, bone morphogenic protein 15 (bmp15), promotes female development by increasing expression of cyp19a1a, an aromatase enzyme that converts androgens to estrogens. The subsequent increase in estrogens is thought to promote differentiation of bipotential somatic cells into granulosa cells. Loss of bmp15 in adults results in female to male sex reversal in zebrafish (26). Similarly, mutations in both foxl2a and foxl2b resulted in 100% male sex bias in adult zebrafish, probably because of increased expression of testes determination genes dmrt1 and amh, genes that are repressed by foxl2 genes (23). However, how these genes interact with AR signaling in zebrafish is not well understood.
Expression of zebrafish ar during gonad development supports a role for its involvement in sex differentiation. During the transition of the bipotential gonad stage into a mature ovary or testis, ar was expressed in both male and female zebrafish, beginning at 1 day postfertilization (dpf) and peaking in expression at ~25 dpf (27, 28). Similarly, ar was upregulated in testes, compared with the ovaries, at the time of sex differentiation (35 dpf), indicating a potential role of AR in these processes (29).
Furthermore, zebrafish, like many teleost species, are vulnerable to androgen exposure during development. Exposure to the antiandrogen flutamide for 48 hours impaired spermatogenesis (30). Exposure to the AR antagonist vinclozolin, during juvenile stages, when sex differentiation occurs, produced a population of adults that were mostly female and exhibited reduced fecundity (31). Similarly, rearing zebrafish in the presence of the AR agonist 17β-trenbolone produced all male adults (32–34). Together, these studies suggest that AR influences sex determination and differentiation in zebrafish.
To investigate the role of AR in zebrafish sexual determination and development, we developed ar mutant zebrafish by using CRISPR-Cas technology and compared secondary sex characteristics, gonad morphology, and expression of sex-related genes between wild-type and mutant adults. Our findings suggest that AR influences sex determination and is necessary for proper organization of the testis in males and for oocyte maturation in females.
Methods
Animal care and husbandry
Zebrafish were housed at the University of Alabama at Birmingham (UAB) Zebrafish Research Facility. All procedures were approved by the UAB Institutional Animal Care and Use Committee. Zebrafish embryos were raised in E3B (60× E3B: 17.2 g NaCl, 0.75 g KCl, 2.9 g CaCl2–H2O, 2.39 g MgSO4 dissolved in 1 L Milli-Q water; diluted to 1× in 9 L Milli-Q water plus 100 μL 0.02% methylene blue) and housed in an incubator at 28.5°C on a 14-hour light, 10-hour dark cycle, until 5 dpf. At 5 dpf they were transferred to tanks on a recirculating water system (Aquaneering, Inc., San Diego, CA). All zebrafish were from the AB strain (35).
Generation of ar guide RNA and Cas9 messenger RNA
Guide RNA (gRNA) was generated against the following ar target sequence: 5′-GGGTACCACTGCTCGTGCGGAGG-3′. Plasmids pT7-gRNA and pT3TS-nCas9n were obtained from Addgene (numbers 46759, 46757) (36). To generate a vector containing ar gRNA, pT7-gRNA was digested simultaneously with BsmBI, BglII, and SalI for 1 hour at 37°C followed by 1 hour at 55°C. Oligonucleotides were synthesized by Invitrogen (5′- TAGGGTACCACTGCTCGTGCGG-3′ and 5′-AAACCCGCACGAGCAGTGGTACCC-3′), hybridized to one another in NEBuffer3 (New England BioLabs, Ipswich, MA) and annealed into digested pT7-gRNA with Quick T4 DNA Ligase (New England BioLabs) as previously described (36). The final vector was confirmed by DNA sequencing, linearized with BamHI, and used as a template to synthesize gRNA with the MegaShortScript T7 Kit (Thermo Fisher Scientific, Waltham, MA). gRNA was purified with the RNA Clean & Concentrator kit (Zymo Research, Irvine, CA). RNA concentration was quantified with a Nanodrop spectrophotometer (Nanodrop ND-1000, Thermo Fisher). Cas9 messenger RNA was generated similarly with plasmid pT3TS-nCas9n as template (36).
Embryo injections
One-cell-stage embryos were injected using glass needles pulled on a Sutter Instruments Fleming/Brown Micropipette Puller model P-97 and a regulated air pressure microinjector (Harvard Apparatus, NY, PLI-90). Each embryo was injected into the yolk with a 1-nL solution of 150 ng/μL of Cas9 messenger RNA, 50 ng/μL of gRNA, and 0.1% phenol red. Injected embryos (F0) were raised to adulthood and crossed to wild-type fish (AB) to generate heterozygous F1 embryos. F1 fish with germline mutations were identified by high-resolution melting curve analysis and DNA sequencing to select mutations predicted to cause loss of functional protein. For DNA sequencing, genomic DNA was amplified with primers 5′-GAGTTTGCTGGTCCCATGGA-3′ and 5′-TCCCGTTTGAGAGGTGCAAA-3′, TA-cloned into vector pCR2 per manufacturer’s instructions (Thermo Fisher) and then sequenced.
Genomic DNA isolation
Individual embryos or tail biopsies from individual adults were placed in 100 μL embryo lysis buffer (10 mM Tris pH 8.3, 50 mM KCl, 0.3% Tween 20) with 1 μL proteinase K (800 U/mL, New England Biolabs) in 96-well plates, one sample per well. Samples were incubated at 55°C for 2 hours (embryos) or 8 hours (adult tail clips) to extract genomic DNA. To inactivate proteinase K, plates were incubated at 98°C for 10 minutes and stored at −20°C.
High-resolution melt curve analysis
Polymerase chain reaction (PCR) and melting curve analysis were performed as described (37). PCR reactions contained 1 μL of LC Green Plus Melting Dye (BioFire Defense, Salt Lake City, UT), 1 μL of Ex Taq Buffer, 0.8 μL of dNTP Mixture (2.5 mM each), 1 μL of each primer (5 μM; 5′-ATACGGCCGAAGTACTGCTC-3′ and 5′-TACGGATGACGGGTCAGCAT-3′), 0.05 μL of ExTaq (Takara Bio USA, Mountain View, CA), 1 μL of genomic DNA, and water up to 10 μL. PCR was performed in a Bio-Rad C1000 Touch thermal cycler, with black/white 96-well plates (Bio-Rad HSP9665; Bio-Rad Laboratories, Hercules, CA). PCR reaction protocol was 98°C for 1 minute, then 34 cycles of 98°C for 10 seconds, 60°C for 20 seconds, and 72°C for 20 seconds, followed by 72°C for 1 minute. After the final step, the plate was heated to 95°C for 20 seconds and then rapidly cooled to 4°C. Melting curves were generated either with a LightScanner HR 96 (Idaho Technology) over a 70°C to 95°C range and analyzed with LightScanner Instrument and Analysis Software (version 2.0.0.1331; Idaho Technology, Inc., Salt Lake City, UT) or with a Bio-Rad CFX96 Real-Time System over a 70°C to 95°C range and analyzed with Bio-Rad CFX Manager 3.1 software (Bio-Rad Laboratories, Inc.).
Secondary sexual characteristics and sex ratios
Heterozygous aruab105/+ adult males and females were bred to each other, offspring were raised to adulthood and genotyped with high-resolution melting curve analysis to examine Mendelian ratios, and secondary sexual characteristics were assayed visually. Zebrafish sex was determined by examining multiple secondary sexual characteristics including body shape, standard length, coloration, and presence of genital papilla, characteristics previously shown to be sex specific (38). Fish were anesthetized in 0.2 mg/mL tricaine for ~2 minutes, then measured for standard length with digital calipers, weighed (wet), examined, and photographed with a Nikon AZ100 microscope equipped with Nikon DS-Fi1 camera (Nikon Instruments, Melville, NY) with ×1 (anal fin) and ×2 (genital papilla) objectives (Fig. 1). Because of the feminized secondary sexual characteristics in male aruab105/uab105 homozygotes, sex was confirmed via gonad dissection.
Figure 1.
Male aruab105/uab105 fish have feminized body shape and anal fin coloration, whereas aruab105/uab105 females display normal secondary sexual characteristics. (A) Male wild-type fish have a slender body shape, (A′) lack a genital papilla, and (A″) have a golden anal fin. (B) The aruab105/uab105 mutants have a rounded body shape, (B′) no genital papilla, (B″) a white anal fin, and an internal testis. (C) Wild-type females and (D) aruab105/uab105 females have a rounded body shape with a prominent protruding abdomen, (C′ and D′) large extended genital papilla (arrows), and (C″ and D″) a white anal fin. A′ and A″ are high-magnification images of boxed areas in (A); remaining images are similarly arranged. Scale bars: (A–D) 1 cm, (A′–D′) 100 μm, (A″’–D″’) 1000 μm.
Breeding and in vitro fertilization
All wild-type and aruab105/105 mutant lines were bred on the same schedule, approximately every 2 weeks, to maintain fertility. To measure fecundity, fertility, and mating success, males and females were set up in breeding tanks with wild-type fish in a 1:2 ratio (mutant to wild-type). In addition, sterility was assessed with in vitro fertilization (IVF) (35). For IVF, mutant and wild-type fish were anesthetized in 0.2 mg/mL tricaine and patted dry, and their abdomen was gently stroked with blunt-ended forceps to elicit excretion of sperm or eggs. A capillary tube and mouth pipette were used to extract sperm, which upon extraction was activated in 200 μL Hanks balanced salt solution. An additional form of IVF was used in which male fish were euthanized in iced 0.2 mg/mL tricaine, and testes were dissected, mixed with 200 μL Hanks balanced salt solution, and minced with a mini–mortar and pestle in a centrifuge tube. Expelled egg clutches from a single female were divided and mixed with 50 μL of extracted testis from both wild-type and mutant testes. Sperm obtained from minced testes were visualized and imaged via differential interference contrast microscopy on a Zeiss Axio Observer.Z1 microscope with a Zeiss Axio MRc5 camera (Carl Zeiss Microscopy GmbH, Jena, Germany) and ×40 objective.
Histology
Whole-body 9-month-old adult wild-type (n = 8, four males and four females) and aruab105/105 mutant fish (n = 7, four females and three males) were selected for histology based on secondary sexual characteristics and IVF results. For examination of gonad developmental stages, wild-type and aruab105/105 juvenile clutch mates were collected at 21, 25, 31, and 38 dpf. Fish were anesthetized in iced 0.2 mg/mL tricaine, decapitated, and cut just above the tail with a razor blade, followed by a 5-day fixation in 4% formaldehyde. Specimens were shipped to HistoWiz Inc. (New York, NY), where they were processed, embedded, sectioned, and stained with hematoxylin and eosin (H&E). Processing of tissues into paraffin blocks was accomplished with an automated Peloris II tissue processor (Leica Biosystems, Buffalo Grove, IL) and embedded in Histoplast PE (Thermo Scientific). Dehydration steps were as follows: 50% ethanol (15 minutes, 45°C), 70% ethanol (15 minutes, 45°C) × 2, 90% ethanol (15 minutes, 45°C), 90% ethanol (30 minutes, 45°C), 100% ethanol (45 minutes, 45°C), 100% xylene (20 minutes, 45°C) × 2, 100% xylene (45 minutes, 45°C), Parablock wax (30 minutes, 65°C) × 2 (Leica Biosystems), and Parablock wax (45 minutes, 65°C). Embedded specimens were cut into 10-μm sections and adhered to charged slides. Slides were heated (65°C, 10 minutes) in an oven to melt paraffin then rehydrated as follows with a Tissue-Tek Prisma (Sakura): 100% xylene (5 minutes) × 2, 100% ethanol (2 minutes) × 2, 95% ethanol (1 minute), deionized (DI) H2O (1 minute), hematoxylin (1 minute), DI H2O (1 minute), defining solution (45 seconds) (Define MX-aq; Leica Biosystems), DI H2O (1 minute), bluing agent (Fisher Scientific), DI H2O (1 minute), 95% ethanol (15 seconds), eosin (5 seconds), 95% ethanol (2 minutes), 100% ethanol (3 minutes) × 2, and 100% xylene (5 minutes) × 2.
Brightfield images of sections were obtained with a Zeiss Axio Observer.Z1 microscope with a Zeiss Axio MRc5 camera with ×10 (oocytes), ×20 (oocytes, testes), and ×40 (testes) objectives. Tiled images of oocytes were captured with 10% overlap, and edges were fused according to the stitching algorithm of Zeiss ZEN 2 blue edition software. Number of oocytes in stage I, II, or III in wild-type and aruab105/105 were counted across nine sections of individual fish (n = 3) and averaged. Statistical analysis of oocyte stages was completed with a one-way analysis of variance (ANOVA) with Tukey post hoc test for multiple comparisons with Prism version 7.0 (GraphPad Software, Inc., La Jolla, CA). A P value of <0.05 was considered significant.
RNA extraction and quantitative reverse transcription PCR
Total RNA was extracted from 9-month-old adult wild-type and aruab105/uab105 zebrafish according to manufacturer’s guidelines for the TRIzol RNA isolation kit (Life Technologies, Carlsbad, CA). Dissected gonads were homogenized individually in 500 μL TRIzol, and total RNA was DNase-treated with a TURBO DNA-Free Kit (Ambion) to remove genomic DNA contamination. RNA was quantified with a NanoDrop ND-1000 ultraviolet-Vis spectrophotometer (Thermo Scientific), and quality was determined with gel electrophoresis.
Expression of genes previously shown to be associated with sex differentiation were examined with quantitative reverse transcription PCR (RT-PCR): cyp19a1a (28), foxl2a, sox9a, amh, dmrt1 (28), and rp113a as a reference gene (17, 39). Primer pairs (Supplemental Table 1 (160.4KB, pdf) ) were tested in an RT-PCR that was incubated at 98°C for 1 minute, followed by 34 cycles of 98°C for 10 seconds, 60°C or 61°C for 20 seconds, and 72°C for 45 seconds, then 72°C for 2 minutes. Products were then run out on a 1% gel to check for product amplification.
Four biological replicates of gonads were collected for each sex and genotype. The Bio-Rad CFX96 Touch Real-Time PCR Detection System and SsoAdvanced Universal SYBR Green Supermix kit (Bio-Rad Laboratories, Inc.) were used for quantitative RT-PCR according to manufacturer recommendations. Biological replicates were run in quadruplicate on 96-well plates, and transcript quantification was measured with CFX Manager version 3.1 software (Bio-Rad Laboratories Inc.). Cq expression values were averaged across technical replicates, primer efficiencies were calculated, and expression fold change values were determined according to the ΔΔCt method (40). A one-way ANOVA was performed on ΔΔCt values with a Tukey test for multiple comparisons to determine significant differences in gene expression between wild-type and mutants.
Results
Generation of zebrafish ar mutants
Using CRISPR-Cas technology (36, 41), we generated mutations in the zebrafish ar gene (nr3c4) upstream of the DNA binding domain (Supplemental Fig. 1 (160.4KB, pdf) ). We identified F1 fish with a 10 base pair (bp) deletion in ar leading to a premature stop codon, predicted to have loss of functional protein (Supplemental Fig. 1 (160.4KB, pdf) ). Mutants were bred to homozygosity and designated as line uab105.
Male aruab105/105 have feminized secondary sexual characteristics but lack a genital papilla
To examine the influence of ar on zebrafish sexual development, we first assayed secondary sexual characteristics: body shape, coloration, and presence of genital papilla (Fig. 1). Females are typically longer than males and have a more rounded abdomen, protruding genital papilla, and white anal fins. In contrast, males appear smaller than females, have a slender body shape, lack a genital papilla, and display a golden anal fin (38).
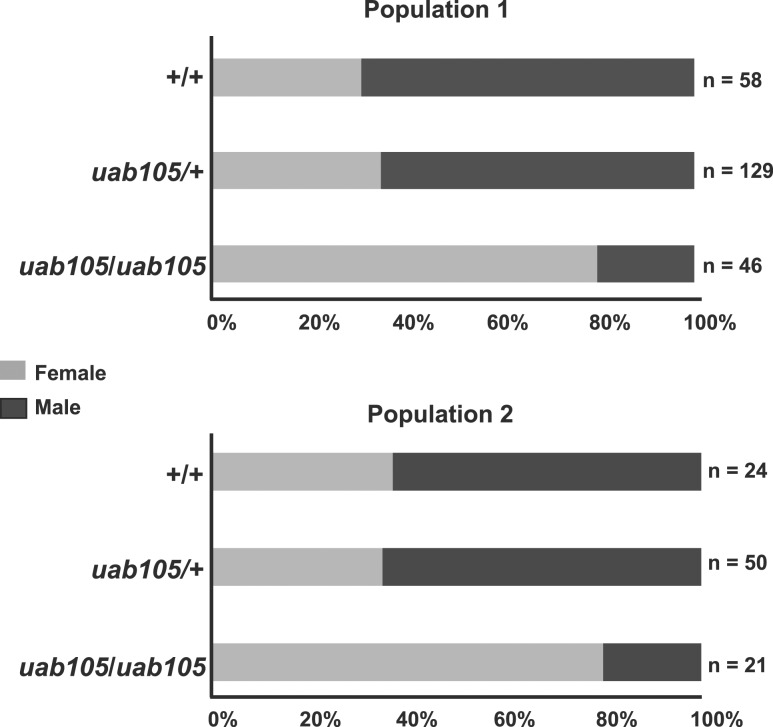
Heterozygous aruab105/+ adult fish exhibited wild-type secondary sex characteristics and were easily identifiable as male or female (data not shown). Therefore, to generate homozygous aruab105/uab105 fish, we mated aruab105/+ adults. Progeny exhibited the expected Mendelian ratios (1:2:1) of homozygous to heterozygous to wild-type (Table 1). All aruab105/uab105 had rounded abdomens typical of females and white anal fins (n = 67, Fig. 1). We observed that a majority of homozygotes had a genital papilla, consistent with wild-type females (n = 54). However, ~20% lacked a genital papilla, consistent with wild-type males, despite exhibiting female-type coloration and rounded abdomens (n = 13, Fig. 1B′). Thus, all aruab105/uab105 adults exhibited female-type rounded abdomen and anal fin coloration, but some lacked a genital papilla.
Table 1.
Mendelian and Sex Ratios of Two Populations Derived From uab105/+
| +/+ | uab105/+ | uab105/uab105 | |
|---|---|---|---|
| Population 1, n (%) | 58 (25%) [40 ♂, 18♀] | 129 (55%) [84 ♂, 45♀] | 46 (20%) [8 ♂, 38♀] |
| Population 2, n (%) | 24 (25%) [15 ♂, 9♀] | 50 (53%) [29 ♂, 21♀] | 21 (22%) [5 ♂, 16♀] |
Each population represents the offspring of harem breeding among male and female uab105/+ heterozygotes. Total number of individual fish within each genotype is indicated, followed by percentage of fish with indicated genotype. Numbers of males and females for each genotype are included in brackets, with sex based on presence of a testis (♂) or ovary (♀).
We hypothesized that the presence of a genital papilla is indicative of an ovary, whereas the lack of a genital papilla correlates to presence of testis. To test this hypothesis, we dissected gonads from aruab105/uab105 adults. We found a perfect correlation between presence of a genital papilla and presence of ovaries (n = 54 fish) and a perfect correlation between absence of genital papilla and presence of testes (n = 13 fish). Therefore, we indicate males and females based on gonadal sex.
We also noticed that the ratio of females to males was biased toward females in aruab105/uab105 compared with heterozygous and wild-type clutch mates (Table 1 and Fig. 2). In our zebrafish colony, wild-type and heterozygous genotypes exhibited a male bias (58% and 68% males in two separate populations), whereas homozygous (aruab105/uab105) genotypes exhibited a strong female bias (76% and 83% female, 17% and 24% male) (Table 1 and Fig. 2).
Figure 2.
The aruab105/uab105 adults have a female sex bias. Percentages of male and female progeny from crosses between aruab105/+ males and females. Offspring were observed to have 1:2:1 Mendelian ratios, but sex ratios were biased, depending on genotype. See Table 1 for details on Mendelian ratio percentages.
Male aruab105/105 fish are unable to release sperm
To evaluate the influence of ar on fertility, we attempted to breed male aruab105/uab105 (fish lacking genital papilla) with female wild-type under harem spawning conditions in three mating trials (one male together with two females, n = 4 mutant males). No homozygous males were successful in breeding with wild-type females under natural spawning conditions. This suggests that mutant males either lack functional sperm, produce functional sperm but cannot release sperm, or produce and release functional sperm but fail to induce females to release oocytes. To test whether aruab105/uab105 produce functional sperm, we attempted to extract sperm from males and fertilize wild-type oocytes in vitro by using standard, nonsurgical procedures (35). After three trials of in vitro fertilization, we extracted functional sperm from 100% of wild-type males (n = 3 fish per trial) but failed to extract sperm from any male aruab105/uab105 fish (n = 10 fish per trial). We failed to extract any liquid, much less sperm, from any ar mutant male fish. We even attempted the extraction while observing the fish under a dissecting microscope and failed to observe any liquid extruded from any mutant male fish in any trial. These results suggest that aruab105/uab105 males are unable to release sperm.
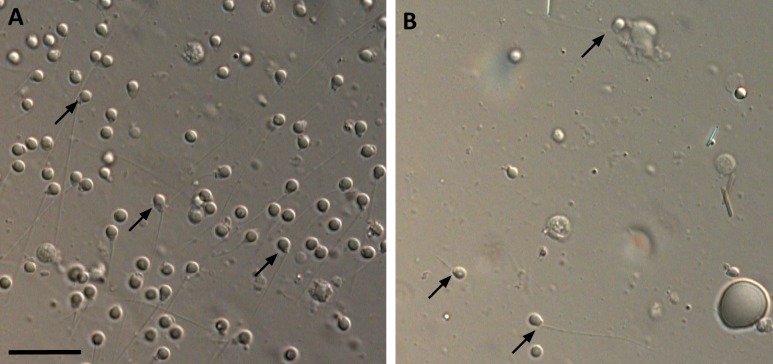
To determine whether aruab105/uab105 produce functional sperm, we dissected testes and immediately visualized them under a microscope, without fixation. We observed sperm with heads and tails in both wild-type and aruab105/uab105 fish (Fig. 3), although mutants had fewer sperm per field of view compared with wild-type.
Figure 3.
aruab105/uab105 testis contains mature sperm. (A) Wild-type and (B) aruab105/uab105 mature sperm (arrows) from dissected testis. Scale bar = 20 μm.
To test whether aruab105/uab105 sperm were able to fertilize oocytes, we mixed aruab105/uab105 testis extracts with wild-type oocytes and assayed the number of fertilized embryos compared with wild-type testis extracts. We found that sperm from dissected, minced testis from aruab105/uab105 were capable of fertilizing wild-type oocytes (Table 2). However, we did notice some within-clutch variability in oocyte survival, because some wild-type and ar mutant testis did not fertilize any oocytes from a single clutch. Fertilized oocytes appeared grossly normal through 4 dpf, at which point we euthanized and then genotyped each larva. All larvae were heterozygous aruab105/+ as expected (n = 24). Our results suggest that aruab105/uab105 males produce but are unable to release functional sperm.
Table 2.
Sperm From Surgically Removed Testis of aruab105/uab105 Can Successfully Fertilize Wild-Type Oocytes In Vitro
| Clutch 1 | Clutch 2 | Clutch 3 | Clutch 4 | Clutch 5 | Clutch 6 | |
|---|---|---|---|---|---|---|
| Wild-type | 0 | 16 | 0 | 4 | 12 | 3 |
| uab105/uab105 | 0 | 0 | 2 | 22 | 0 | 0 |
Each wild-type egg clutch was divided and exposed to surgically removed and minced testis from either one wild-type or one mutant fish. Number of 4-dpf larvae derived from wild-type or uab105/uab105 sperm are indicated for each clutch of oocytes.
Male aruab105/uab105 fish have disorganized seminiferous tubules and aberrant cyst formation
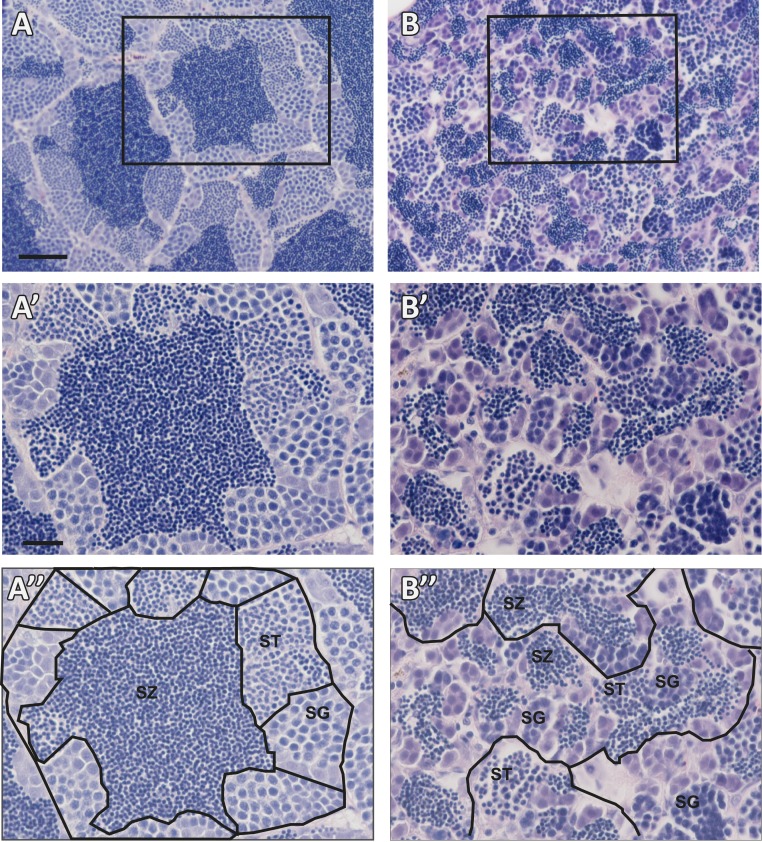
Because male aruab105/uab105 were unable to release sperm, we assayed testes for morphological defects. We performed H&E staining on tissue sections from aruab105/uab105 testes and observed disorganized seminiferous tubules compared with wild-type testes (Fig. 4; representative images from n = 3 fish). In wild-type testes, we observed distinct seminiferous tubules, surrounded by a basement membrane, filled with developing sperm organized into readily demarcated cysts with mature sperm (spermatozoa) congregated in the lumen cavity, consistent with previous findings (42). In contrast, aruab105/uab105 males lacked seminiferous tubule structure and exhibited disorganized cyst formation with no central pool of spermatozoa. Sperm of similar developmental stages (based on diameter of nucleus) were clustered together in cysts, but the cysts were not clustered into tubules as in wild-type testes (Fig. 4, compare A″ and B″). Additionally, sperm cysts were smaller in mutants compared with wild-type. Our results suggest that AR is necessary for proper testicular organization in zebrafish.
Figure 4.
aruab105/uab105 testes are disorganized and lack seminiferous tubule structure. H&E staining of adult male (A) wild-type and (B) aruab105/uab105 sectioned testis. Boxed areas in (A) and (B) represent (A′), (A″), (B′), and (B″), respectively. Wild-type testis contains spherical seminiferous tubules surrounded by a basement membrane, with cysts of developing sperm surrounding mature sperm in the tubule lumen, whereas aruab105/uab105 males lack seminiferous tubule organization and cyst structure. (A″, B″) Tubules and cysts from (A′) and (B′) are outlined in black, and spermatogenesis stages are indicated: spermatogonia (SG), spermatids (ST), spermatozoa (SZ). Scale bars: (A, B) 50 μm; (A′, B′, A″, B″) 20 μm.
Female aruab105/105 fish have lower fecundity and fewer mature oocytes
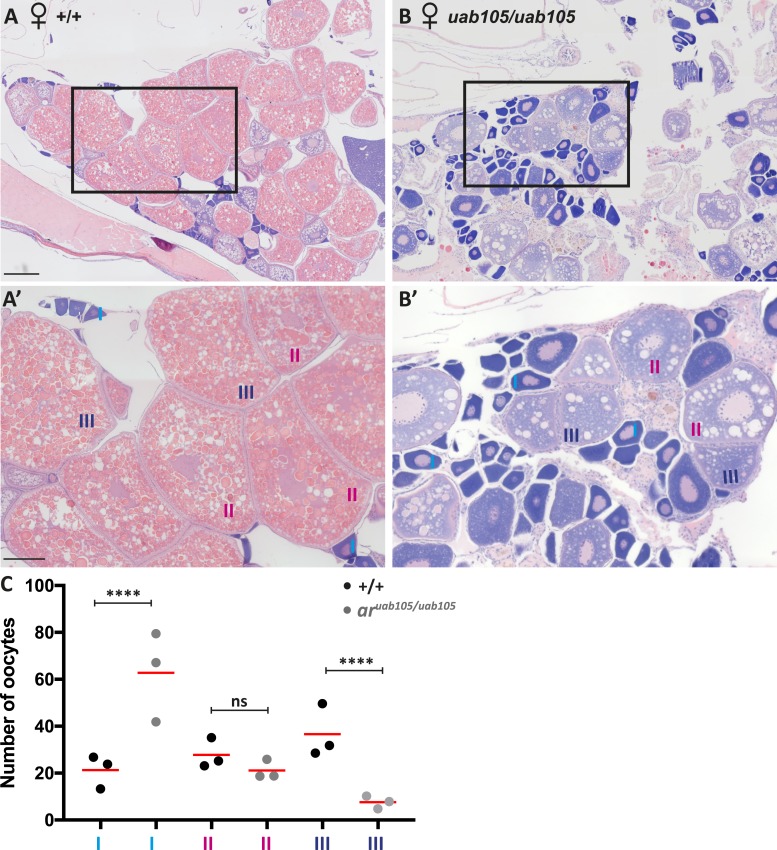
In contrast to aruab105/uab105 males, aruab105/uab105 females were fertile and able to breed with wild-type and aruab105/+ zebrafish. However, mutant females spawned on average 18% of the time (n = 13 fish, three trials) and released 16.14 ± 5.12 oocytes [mean ± standard deviation (SD)], whereas wild-type females spawned 50% of the time (n = 10 fish, three trials) and released 108.2 ± 46.88 oocytes. This indicates that mutant females have decreased fecundity and release fewer oocytes on average, compared with wild-type clutch mates. To determine whether there were morphological defects in mutant ovaries contributing to reduce fecundity, we performed H&E staining on tissue sections from wild-type and aruab105/uab105 ovaries. The overall architecture and organization of ovaries were similar between wild-type and mutant females (Fig. 5; representative images from n = 4 fish per genotype). Ovaries from wild-type and mutant fish contained oocytes of different stages, from less mature stage I (primary growth stage) through stage II (cortical alveolus stage) and mature (vitellogenic) stage III oocytes. Stages were determined by the appearance of variably sized cortical alveoli, prominence of the vitelline envelope, and accumulation of yolk proteins (43). However, the ratio of stage I to stage III oocytes was different, with wild-type containing more stage I oocytes than stage III (Fig. 5C). Wild-type fish contained on average 21.1 ± 7.4 stage I oocytes and 36.2 ± 11.4 stage III oocytes, compared with 62.8 ± 18.9 stage I oocytes and 7.6 ± 2.5 in mutants (P = 0.0001, ANOVA with Tukey test for multiple comparisons, n = 3 fish per genotype, 9 tissue sections analyzed per fish, mean ± SD). There was no significant difference in stage II oocytes between wild-type and aruab105/uab105 mutants (27.5 ± 6.4 vs 21.1 ± 4.0, P = 0.12). These results indicate that AR influences oocyte maturation in zebrafish.
Figure 5.
aruab105/uab105 ovaries have fewer mature oocytes than do wild-type ovaries. H&E staining of adult female (A) wild-type and (B) aruab105/uab105 ovary sections. (A′, B′) High-magnification images of boxed areas in (A) and (B). Wild-type ovaries consist of a majority of mature late-stage III followed by stage II oocytes, with stage I oocytes in less abundance. aruab105/uab105 ovaries contain primarily early-staged I and II and almost no stage III oocytes. Scale bar: (A, B) 200 μm, (A′, B′) 50 μm. (C) Quantification of oocyte number according to stage in wild-type and mutant ovaries. Each dot represents average number of oocytes per histological section per fish (n = 3 fish per genotype, 9 sections per fish). Significance is indicated with asterisks (****P < 0.0001; ns = not significant) from one-way ANOVA.
The aruab105/uab105 mutants develop histologically normal juvenile gonads
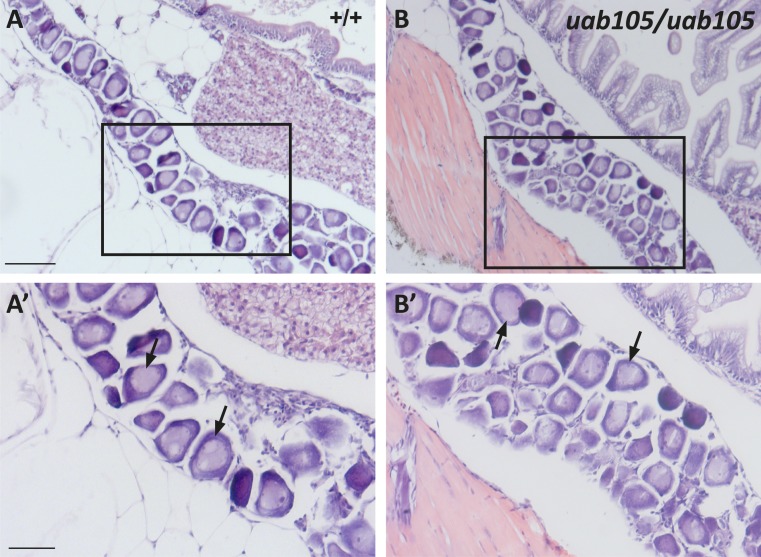
The observation that mature gonads are abnormal in ar mutants suggests that the timing of sexual differentiation could be altered. We examined tissue sections from aruab105/uab105 mutants during the crucial period of sexual differentiation, between 21 and 38 dpf (14). At 21 and 25 dpf, we were unable to identify gonadal tissue in wild-type or aruab105/uab105 by H&E staining (data not shown, n = 5 fish per genotype). At 31 dpf, we observed a thin ribbon of gonad tissue containing immature oocytelike cells, characteristic of a juvenile, bipotential zebrafish gonad (14), in wild-type and aruab105/uab105 (Fig. 6). We conclude that for zebrafish housed in our facility, the juvenile, bipotential gonad developed between 26 and 31 dpf.
Figure 6.
Wild-type and aruab105/105 mutants develop a juvenile gonad at 31 dpf. H&E staining of (A) wild-type and (B) aruab105/uab105 gonad at 31 dpf. (A′, B′) High-magnification images of boxed area in (A) and (B), respectively. Clusters of immature ovarylike cells [black arrows in (A′) and (B′)] are present in juvenile gonads of both wild-type and mutants (n = 5 per genotype).
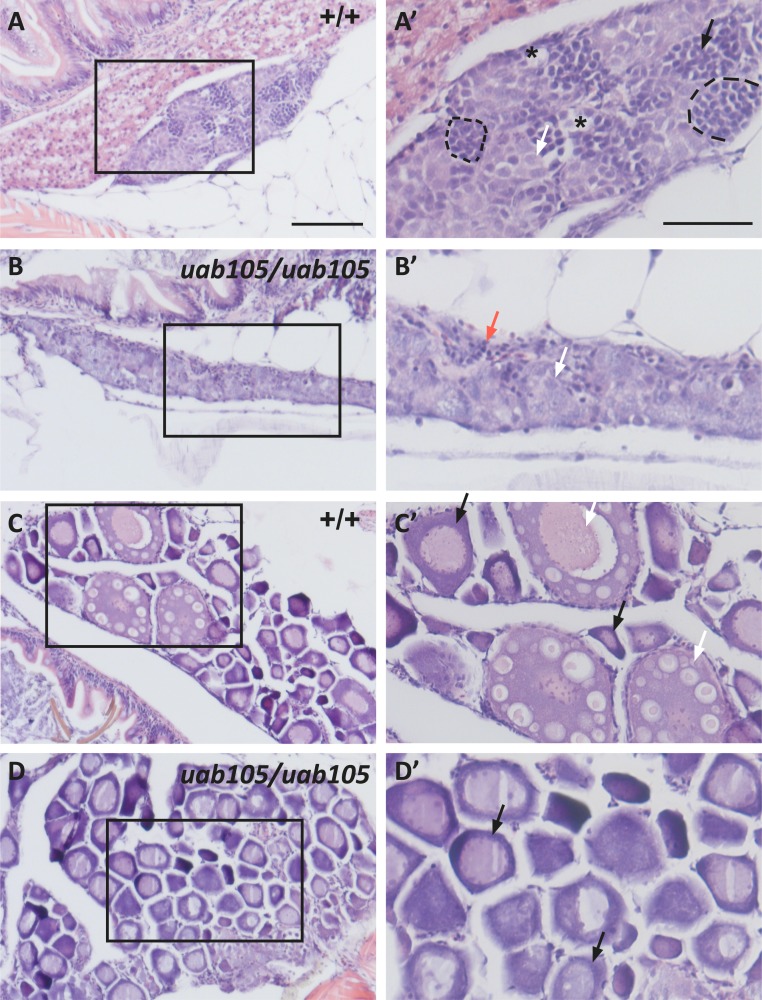
At 38 dpf, both wild-type and aruab105/uab105 mutants exhibited evidence of gonad differentiation (Fig. 7, n = 5 fish per genotype). In four wild-type fish, we observed spermatogonia and spermatids and overall tissue architecture indicative of testes, evidence of a differentiated gonad (Fig. 7A). In one wild-type fish, we observed ovaries containing stage I and II oocytes, demonstrating development beyond the juvenile gonad, which contains no stage II oocytes. Similarly, in 2 aruab105/uab105 mutants we observed testes containing spermatogonia and stroma cells (Fig. 7B), indicative of a differentiated testes and not a juvenile, bipotential gonad (15). However, three aruab105/uab105 mutants exhibited gonads containing only stage I oocytes (Fig. 7D). We were unable to determine whether these gonads are juvenile (and presumably still bipotential) or whether the immature oocytes represent ovaries at the earliest stage of differentiation. We conclude that AR is not necessary for formation of the juvenile gonad.
Figure 7.
Wild-type and aruab105/105 mutants begin gonad differentiation by 38 dpf. H&E staining of (A) wild-type testes, (B) aruab105/uab105 mutant testes, (C) wild-type ovaries, and (D) aruab105/uab105 mutant ovaries at 38 dpf (n = 5 per genotype). High-magnification images of boxed areas in (A), (B), (C), and (D) are shown in (A′), (B′), (C′), and (D′), respectively. (A and A′) Wild-type testes appear recently differentiated because early stages of spermatogonia (white arrow) and spermatids (black arrow) of spermatogenesis are grouped into cysts (outlined), and empty lumens (asterisks) are void of mature spermatozoa. (B and B′) Mutant testes appear as early-staged testes that lack oocytelike cells representative of bipotential gonads. Mutant testes contain stroma cells (red arrows) and clusters of spermatogonia (black arrows) with no spermatids or spermatozoa present. (C and C′) Wild-type ovaries consist of stage I (black arrow) and stage II (white arrow) oocytes, with no mature (stage III) oocytes present. (D and D′) Mutant ovaries appear to have only stage I oocytes present (black arrows) and lack stage II or stage III oocytes, suggesting a delay in oocyte maturation.
The aruab105/uab105 mutants display abnormal expression of sexually dimorphic genes associated with gonad function
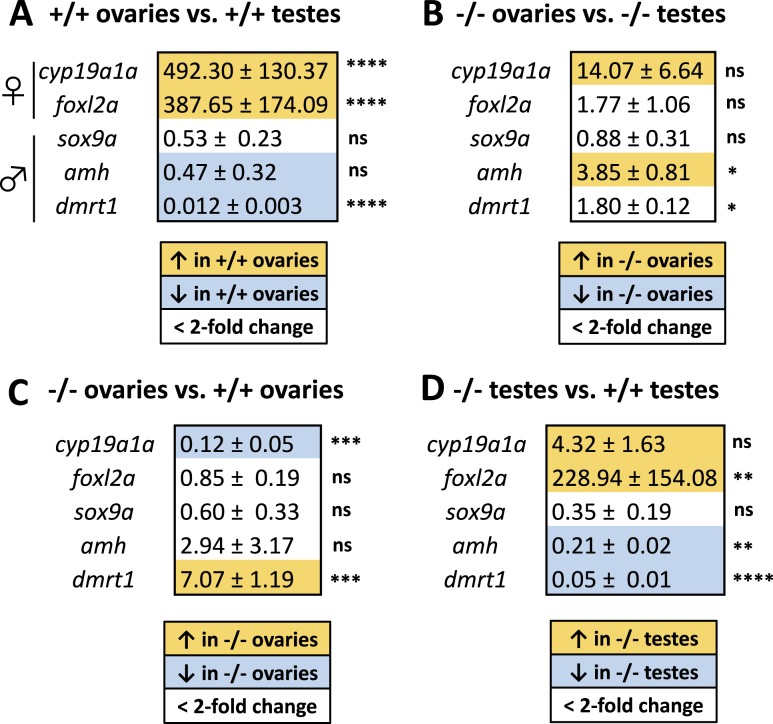
To determine whether nuclear AR acts upstream or downstream of genes known to regulate gonad function in zebrafish, we used a candidate approach and assayed the expression of genes associated with gonad function: cyp19a1a, foxl2a, amh, sox9a, and dmrt1 (17, 21, 23, 24, 26). We compared gene expression levels by using quantitative RT-PCR in replicate ovaries and testes dissected from adult wild-type and aruab105/uab105 mutants (Fig. 8, n = 4 gonads of each sex per genotype) and used the reference gene rpll3a, which is expressed in all tissues, as a control (39). Expression of two genes associated with adult ovary function, cyp19a1a and foxl2a, was highly upregulated in wild-type ovaries compared with wild-type testes, 492.30 ± 130.37 (P = < 0.0001) and 387.65 ± 174.09 (P = < 0.0001) (expression fold change ± SD, Fig. 8A), consistent with previous studies (21). Expression of two genes associated with adult testis function were downregulated in wild-type ovaries compared with wild-type testes: amh (0.47 ± 0.32, P < 0.0001) and dmrt1 (0.012 ± 0.003, P = 0.06) (Fig. 8A), consistent with previous studies (20, 44).
Figure 8.
Quantitative RT-PCR of genes associated with sexual determination and development in wild-type and aruab105/uab105 zebrafish. (A) Expression fold change of indicated genes in wild-type (+/+) ovaries compared with wild-type testes. (B) Expression fold change of aruab105/uab105 mutant (−/−) ovaries compared with mutant testes. (C) aruab105/uab105 mutant ovaries compared with wild-type ovaries and (D) aruab105/uab105 mutant testes compared with wild-type testes. Expression fold change values ± SD are shaded with yellow, indicating an upregulation, blue representing a downregulation, and white representing less than a twofold change up or down. (C) Mutant ovaries show a downregulation in cyp19a1a and an upregulation in dmrt1 compared with wild-type ovaries, whereas (D) mutant testes show an upregulation in cyp19a1a and foxl2a and a downregulation of amh and drmt1 compared with wild-type testes. ****P ≤ 0.0001; ***P ≤ 0.001; **P ≤ 0.01; *P ≤ 0.05; P > 0.05, ANOVA with Tukey test for multiple comparisons. ns, not significant.
In contrast, ar mutants exhibited dysregulation of sexually dimorphic genes. We found that cyp19a1a was not significantly differentially expressed in mutant ovaries compared with mutant testes (14.07 ± 6.64, P = 0.9, Fig. 8B), whereas cyp19a1a was upregulated 492-fold in wild-type ovaries compared with wild-type testes (P < 0.0001, Fig. 8A). This lack of upregulation in mutant ovaries compared with mutant testes could occur because the ar mutant ovary expresses less cyp19a1a than wild-type ovary. Mutant ovaries expressed eightfold lower levels of cyp19a1a compared with wild-type ovaries (0.12 ± 0.05, P = 0.0002; Fig. 8C). We observed a similar pattern with foxl2a, a gene whose expression is normally enriched in wild-type ovaries. Mutant testes exhibited a 229-fold increase in foxl2a expression compared with wild-type testes (P = 0.003) (Fig. 8D).
Expression of dmrt1, a gene enriched in wild-type testes, was upregulated sevenfold in ar mutant ovaries compared with wild-type ovaries (P = 0.0001) and downregulated 20-fold in mutant testes compared with wild-type testes (P < 0.0001). Similarly, amh, a gene normally enriched in testes (20, 45), was upregulated 3.9-fold in ar mutant ovaries compared with mutant testes (P = 0.01) and downregulated 4.8-fold in ar mutant testes compared with wild-type testes (P = 0.002). Expression of sox9a, a gene normally enriched in testes (17, 20), did not change more than twofold in any comparison and therefore was not significantly differentially expressed, suggesting that sox9a expression is not affected by AR. Our results suggest that gene expression is partially feminized in aruab105/uab105 testes and partially masculinized in aruab105/uab105 ovaries.
Discussion
AR influences zebrafish sex determination but not early gonad differentiation
Our data suggest that nuclear AR influences male sex determination in zebrafish, because the majority of aruab105/uab105 mutant fish develop as females. Considering that exposure to potent androgens was reported to fully masculinize zebrafish, it was not surprising that AR plays a role in sex determination (26, 32–34). The influence of AR on sex determination is also consistent with pharmacological studies in other fish species. For example, female sex-biased populations were also reported in juvenile guppies exposed to AR antagonists (46).
The presence of histologically normal juvenile gonads in wild-type and ar mutants at 31 dpf (Fig. 7) suggests that AR is not necessary for development of the juvenile gonad. However, examination of gonads at 38 dpf and in adults indicates that AR may be involved in germ cell maturation. At 38 dpf, mutant ovaries consisted of only stage I oocytes, compared with wild-type ovaries that had both stage I and II oocytes. This disparity continued into adulthood, where mutant ovaries had a higher ratio of immature (stage I) to mature (stage III) oocytes compared with wild-type. A more thorough examination of later developmental time points is needed to confirm timing of germ cell maturation in wild-type and aruab105/105 mutant gonads. Therefore, we conclude that AR influences sex determination but is not necessary for initial differentiation of the juvenile gonad. Although the gonad at 31 dpf in ar mutants appears histologically similar to the wild-type bipotential gonad, whether the juvenile ar mutant gonad is bipotential to the same extent as the wild-type juvenile gonad remains an open question.
Studies in AR mutant mice do not report sex-biased populations. There is a clear disparity in the role of AR in sex determination between mice and zebrafish, probably a result of laboratory strains of zebrafish lacking sex chromosomes and sex determination genes (12, 13, 47). In contrast to the laboratory AB strain of zebrafish studied here, evidence suggests that wild zebrafish contain a sex determination region on chromosome 4 (12). Similarly, in medaka fish, the presence of the gene dmy specifies male sex (48). It will be interesting to explore how AR influences sex determination and gonad development and function in wild zebrafish and in medaka.
AR regulates zebrafish testicular organization and structure
In wild-type zebrafish testes, distinct seminiferous tubules are filled with developing sperm organized into spermatogenic cysts, with mature sperm (spermatozoa) centralized in the lumen cavity (42). We found that testes of aruab105/105 were structurally disorganized, with a lack of seminiferous tubules containing developing sperm surrounding a lumen filled with mature spermatozoa (Fig. 4). Although testes lacked the structured tubule formation of wild-type, they contained all stages of spermatogenesis, including mature and functional spermatozoa.
Our results indicate the aruab105/105 were infertile because although they produce functional sperm, they could not release sperm. The reason could be improper formation of the duct that transports sperm to the cloaca or a blockage in the duct. Additionally, it is possible that AR could regulate male mating behaviors, whereby ar mutants may be unable to properly court females and elicit oocyte release. While this manuscript was being prepared, another ar mutant zebrafish was reported (49). The authors found that homozygous ar mutant males exhibited reduced courtship behavior compared with wild-type. However, they did not examine or report any reproductive phenotypes and noted that their mutant males were fertile. We speculate that this is because the mutation reported in Yong et al. (49) occurs close to the start codon, leading to an alternatively spliced transcript that produces a largely functional AR protein. This disparity in target site mutation could explain alterative phenotypes, emphasizing the importance of examining multiple alleles when generating mutational knockouts.
AR mutant male phenotypes in zebrafish and mice
Defects in secondary sex characteristics and testis organization are conserved between AR mutant zebrafish and mice. AR mutant male mice exhibit female secondary sex characteristics, such as a shorter external genitoanal distance and lower body weight (more similar to females) (8). Similarly, male ar mutant zebrafish exhibit female body shape and anal fin coloration. Both AR mutant mice and zebrafish exhibit abnormal testis organization, with mutants from both species exhibiting smaller testes that were thinner and less cellular compared with wild-type (8).
AR mutant mice had impaired spermatogenesis, with developing sperm arrested at the pachytene spermatocyte stage, and lacked spermatids and mature spermatozoa (8, 9). The presence of mature sperm in ar mutant zebrafish is in contrast to findings in mice. This finding could indicate that AR has different roles in spermatogenesis between mammals and zebrafish.
One possible explanation for differences in AR regulation of spermatogenesis between mice and zebrafish is that other receptors may be involved in sperm maturation in zebrafish. ZIP9 (slc39a9), a member of the zinc transporter subfamily, was shown to bind androgens with high affinity in Atlantic croaker (50). In addition, a calcium and amino acid sensing G-protein coupled receptor has been shown to transduce nongenomic effects of androgens in mice (51). Both of these genes are conserved in zebrafish and therefore could be involved in androgen-dependent testis determination and spermatogenesis. Alternatively, the presence of testes and functional sperm in aruab105/105 mutant males could indicate that the uab105 allele is hypomorphic and demonstrates a partial but not complete loss of AR function. Because the targeted mutation in the ar gene created a frameshift and premature stop codon before the DNA and ligand binding domains (Supplemental Fig. 1 (160.4KB, pdf) ), the uab105 mutant is expected to be a null allele.
AR-dependent oocyte maturation and fecundity in zebrafish are consistent with findings in frog and mouse
Our results indicate that a loss of AR function causes an oocyte maturation defect in zebrafish because ar mutant ovaries contained majority stage I oocytes and few mature stage III oocytes. Loss of AR leading to decreased oocyte maturation is consistent with results in frogs, where androgens regulate oocyte maturation (52). In Xenopus laevis frogs, oocyte maturation in vitro was promoted by testosterone and blocked by an AR antagonist, suggesting that androgens act through AR to induce oocyte maturation (52). Similarly in mice, testosterone induced maturation of oocytes arrested in prophase I of meiosis (11). Our results also indicate that a loss of AR function causes decreased fecundity in zebrafish, because ar mutant females spawned less often and released fewer eggs than wild-type females. This result is supported by findings in AR mutant mice, which showed decreased fecundity and fewer ovarian follicle cells compared with wild-type mice (10, 53). Therefore, we conclude that a loss of AR function causes decreased fecundity due to an oocyte maturation defect.
AR gene networks
The aruab105/105 mutant testes exhibited partially feminized gene expression patterns, similar to wild-type ovaries. Upregulation of foxl2a in aruab105/105 mutant testes compared with wild-type testes suggests that AR represses foxl2a expression in adult testes, which may represent a mechanism of testicular maintenance, because foxl2a promotes ovarian function (23). In contrast, downregulation of dmrt1 in aruab105/105 mutant testes compared with wild-type testes suggests that AR upregulates dmrt1 expression in adult testes. Consistent with this idea, dmrt1 mutant zebrafish displayed disorganized testes that lacked seminiferous tubule structure, suggesting that AR regulates testicular organization and structure via upregulation of dmrt1 (21).
Whereas dmrt1 expression is reduced in ar mutant testes compared with wild-type, dmrt1 expression is increased in ar mutant ovaries compared with wild-type ovaries. This suggests that in wild-type ovaries, AR downregulates dmrt1 expression, whereas in wild-type testes, AR upregulates dmrt1 expression. The molecular and cellular mechanisms by which AR upregulates one gene in male gonads but downregulates the same gene in female gonads is not known.
Additionally, our results do not address whether ar regulates dmrt1, foxl2a, amh, or cyp19a1a expression directly or indirectly. Evidence suggests that dmrt1, foxl2a, and cyp19a1a interact with each other to regulate gonad function. Because dmrt1 downregulates foxl2a expression in zebrafish testes (21), loss of dmrt1 expression in ar mutant testes may be partially responsible for increased levels of foxl2a. In this scenario, ar regulates dmrt1, and dmrt1 regulates foxl2a, but ar does not regulate foxl2a directly. Similarly, because dmrt1 upregulates expression of amh in wild-type zebrafish testes (21, 24), downregulation of dmrt1 in ar mutant testes could drive downregulation of amh. These network interactions are modeled in Fig. 9.
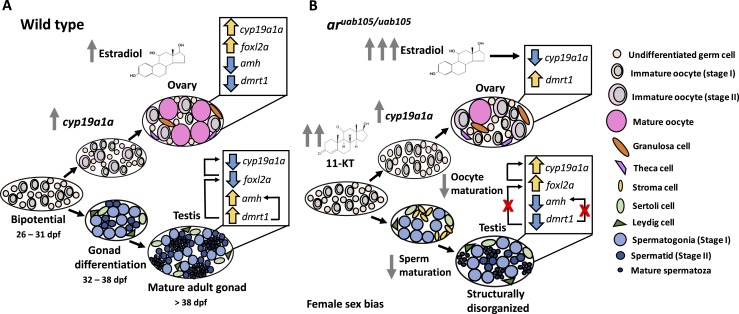
Figure 9.
Model of gonad development in aruab105/uab105 compared with wild-type. (A) Wild-type and (B) aruab105/uab105 mutants develop juvenile gonads at 31 dpf. (A) Wild-type juvenile gonads can become ovaries or testes. Testis development is associated with increased levels of androgens and increased AR signaling. In the mature testes, AR upregulates dmrt1, which then upregulates amh and downregulates ovary-enriched genes foxl2a and cyp19a1a. Ovary development is associated with increased levels of cyp19a1a (the aromatase enzyme that converts androgens to estrogens), leading to increased levels of estrogens (e.g., estradiol), reduced levels of androgens, increased estrogen receptor signaling, and decreased AR signaling. Reduced AR signaling means dmrt1 and amh are not upregulated, relieving the repression of foxl2a and cyp19a1a. (B) The majority of juvenile gonads in aruab105/uab105 develop into ovaries, because of reduced AR signaling that normally promotes testes development. The absence of ARs increases the availability of androgens as cyp19a1a substrates, leading to higher estrogen levels compared with wild-type. In mature ovaries, increased estrogen levels downregulate cyp19a1a and repress oocyte maturation. It is not clear what accounts for dmrt1 upregulation in mutant ovaries compared with wild-type. In the few ar mutants that develop testes, the absence of AR signaling means dmrt1 and amh are not upregulated, allowing increased expression of foxl2a and cyp19a1a.
Amh is also an important regulator of sexual determination in zebrafish, because amh mutant zebrafish displayed a female-biased sex ratio (24). Male amh mutants developed feminized secondary sexual characteristics, including enlarged abdomen and whiter anal fins (24), similar to the ar male mutant phenotypes reported here. The similarities in these mutant phenotypes suggest that AR regulates secondary sexual characteristic through regulation of amh.
The aruab105/105 mutant ovaries showed expression patterns more closely aligned with those of wild-type testes. Expression of dmrt1 was upregulated, whereas cyp19a1a was downregulated. Because estradiol levels were shown to downregulate cyp19a1a in adult zebrafish (54), and because we found that cyp19a1a expression is reduced in ar mutant ovaries compared with wild-type ovaries, it is tempting to speculate that estrogen levels are increased in ar mutant ovaries compared with wild-type. Increased estrogen levels delay oocyte maturation in zebrafish (55), which could explain the oocyte maturation phenotype in aruab105/105 mutant ovaries (Fig. 9). There is currently no assay to reliably detect estrogen levels from zebrafish ovaries.
Acknowledgments
We thank R. Swanson and J. L. King for excellent technical assistance, J. M. Parant for help with high-resolution melting curve analysis, S. N. Romano, J. P. Souder, and H. E. Edwards for support and suggestions, and S. C. Farmer and staff at the UAB Zebrafish Research Facility for animal care.
Financial Support: This work was funded by National Institutes of Health (National Institute of General Medical Sciences Grant K12GM088010 to C.M.C. and National Institute of Environmental Health Sciences Grant R01ES026337 to D.A.G.); by a Faculty Research Grant from Roanoke College (to C.S.L.); and by startup funds from UAB and the Department of Pharmacology & Toxicology.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- amh
- anti-Müllerian hormone
- ANOVA
- analysis of variance
- AR
- androgen receptor
- bmp15
- bone morphogenic protein 15
- DI
- deionized
- dmrt1
- doublesex- and mab-3-related transcription factor 1
- dpf
- day(s) postfertilization
- foxl2a
- forkhead box L2a
- gRNA
- guide RNA
- H&E
- hematoxylin and eosin
- IVF
- in vitro fertilization
- PCR
- polymerase chain reaction
- PGC
- primordial germ cell
- RT-PCR
- reverse transcription polymerase chain reaction
- SD
- standard deviation
- sox9a
- sry-related HMG box gene 9a
- UAB
- University of Alabama at Birmingham.
References
- 1.Brinkmann AO, Faber PW, van Rooij HC, Kuiper GG, Ris C, Klaassen P, van der Korput JA, Voorhorst MM, van Laar JH, Mulder E, Trapman J. The human androgen receptor: domain structure, genomic organization and regulation of expression. J Steroid Biochem. 1989;34(1–6):307–310. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann AO, Klaasen P, Kuiper GG, van der Korput JA, Bolt J, de Boer W, Smit A, Faber PW, van Rooij HC, Geurts van Kessel A, Voorhorst MM, Mulder E, Trapma J.. Structure and function of the androgen receptor. Urol Res. 1989;17(2):87–93. [DOI] [PubMed] [Google Scholar]
- 3.Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240(4850):324–326. [DOI] [PubMed] [Google Scholar]
- 4.Lubahn DB, Joseph DR, Sar M, Tan J, Higgs HN, Larson RE, French FS, Wilson EM. The human androgen receptor: complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol Endocrinol. 1988;2(12):1265–1275. [DOI] [PubMed] [Google Scholar]
- 5.Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240(4850):327–330. [DOI] [PubMed] [Google Scholar]
- 6.Tilley WD, Marcelli M, Wilson JD, McPhaul MJ. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci USA. 1989;86(1):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trapman J, Klaassen P, Kuiper GG, van der Korput JA, Faber PW, van Rooij HC, Geurts van Kessel A, Voorhorst MM, Mulder E, Brinkmann AO. Cloning, structure and expression of a cDNA encoding the human androgen receptor. Biochem Biophys Res Commun. 1988;153(1):241–248. [DOI] [PubMed] [Google Scholar]
- 8.Yeh S, Tsai M-Y, Xu Q, Mu X-M, Lardy H, Huang K-E, Lin H, Yeh S-D, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues [published correction appears in Proc Natl Acad Sci USA. 2002;99(23):15245] Proc Natl Acad Sci USA. 2002;99(21):13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101(5):1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto T, Shiina H, Kawano H, Sato T, Kato S. Androgen receptor functions in male and female physiology. J Steroid Biochem Mol Biol. 2008;109(3–5):236–241. [DOI] [PubMed] [Google Scholar]
- 11.Gill A, Jamnongjit M, Hammes SR. Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol. 2004;18(1):97–104. [DOI] [PubMed] [Google Scholar]
- 12.Wilson CA, High SK, McCluskey BM, Amores A, Yan YL, Titus TA, Anderson JL, Batzel P, Carvan MJ III, Schartl M, Postlethwait JH. Wild sex in zebrafish: loss of the natural sex determinant in domesticated strains. Genetics. 2014;198(3):1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR, Orban L. Polygenic sex determination system in zebrafish. PLoS One. 2012;7(4):e34397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi T. Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bull Fac Fish Hokkaido Univ. 1977;28:57–65. [Google Scholar]
- 15.Uchida D, Yamashita M, Kitano T, Iguchi T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol. 2002;205(Pt 6):711–718. [DOI] [PubMed] [Google Scholar]
- 16.Tzung K-W, Goto R, Saju JM, Sreenivasan R, Saito T, Arai K, Yamaha E, Hossain MS, Calvert ME, Orbán L. Early depletion of primordial germ cells in zebrafish promotes testis formation [published correction appears in Stem Cell Reports. 2015;5(1):156]. Stem Cell Reports. 2015;4(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegfried KR, Nüsslein-Volhard C. Germ line control of female sex determination in zebrafish. Dev Biol. 2008;324(2):277–287. [DOI] [PubMed] [Google Scholar]
- 18.Dranow DB, Tucker RP, Draper BW. Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Dev Biol. 2013;376(1):43–50. [DOI] [PubMed] [Google Scholar]
- 19.Slanchev K, Stebler J, de la Cueva-Méndez G, Raz E. Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci USA. 2005;102(11):4074–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez-Marí A, Yan YL, Bremiller RA, Wilson C, Cañestro C, Postlethwait JH. Characterization and expression pattern of zebrafish Anti-Müllerian hormone (Amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr Patterns. 2005;5(5):655–667. [DOI] [PubMed] [Google Scholar]
- 21.Webster KA, Schach U, Ordaz A, Steinfeld JS, Draper BW, Siegfried KR. Dmrt1 is necessary for male sexual development in zebrafish. Dev Biol. 2017;422(1):33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau ES-W, Zhang Z, Qin M, Ge W. Knockout of zebrafish ovarian aromatase gene (cyp19a1a) by TALEN and CRISPR/Cas9 leads to all-male offspring due to failed ovarian differentiation. Sci Rep. 2016;6(1):37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y-J, Wang Y, Li Z, Zhou L, Gui J-F. Sequential, divergent, and cooperative requirements of Foxl2a and Foxl2b in ovary development and maintenance of zebrafish. Genetics. 2017;205(4):1551–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Q, Mei J, Li Z, Zhang X, Zhou L, Gui J-F. Distinct and cooperative roles of amh and dmrt1 in self-renewal and differentiation of male germ cells in zebrafish. Genetics. 2017;207(3):1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang EF-L, Yan Y-L, Guiguen Y, Postlethwait J, Chung BC. Two Cyp19 (P450 aromatase) genes on duplicated zebrafish chromosomes are expressed in ovary or brain. Mol Biol Evol. 2001;18(4):542–550. [DOI] [PubMed] [Google Scholar]
- 26.Dranow DB, Hu K, Bird AM, Lawry ST, Adams MT, Sanchez A, Amatruda JF, Draper BW. Bmp15 is an oocyte-produced signal required for maintenance of the adult female sexual phenotype in zebrafish. PLoS Genet. 2016;12(9):e1006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelick DA, Watson W, Halpern ME. Androgen receptor gene expression in the developing and adult zebrafish brain. Dev Dyn. 2008;237(10):2987–2995. [DOI] [PubMed] [Google Scholar]
- 28.Jørgensen A, Andersen O, Bjerregaard P, Rasmussen LJ. Identification and characterisation of an androgen receptor from zebrafish Danio rerio. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146(4):561–568. [DOI] [PubMed] [Google Scholar]
- 29.Hossain MS, Larsson A, Scherbak N, Olsson P-E, Orban L. Zebrafish androgen receptor: isolation, molecular, and biochemical characterization. Biol Reprod. 2008;78(2):361–369. [DOI] [PubMed] [Google Scholar]
- 30.Martinović-Weigelt D, Wang R-L, Villeneuve DL, Bencic DC, Lazorchak J, Ankley GT. Gene expression profiling of the androgen receptor antagonists flutamide and vinclozolin in zebrafish (Danio rerio) gonads. Aquat Toxicol. 2011;101(2):447–458. [DOI] [PubMed] [Google Scholar]
- 31.Lor Y, Revak A, Weigand J, Hicks E, Howard DR, King-Heiden TC. Juvenile exposure to vinclozolin shifts sex ratios and impairs reproductive capacity of zebrafish. Reprod Toxicol. 2015;58:111–118. [DOI] [PubMed] [Google Scholar]
- 32.Larsen MG, Baatrup E. Functional behavior and reproduction in androgenic sex reversed zebrafish (Danio rerio). Environ Toxicol Chem. 2010;29(8):1828–1833. [DOI] [PubMed] [Google Scholar]
- 33.Orn S, Yamani S, Norrgren L. Comparison of vitellogenin induction, sex ratio, and gonad morphology between zebrafish and Japanese medaka after exposure to 17alpha-ethinylestradiol and 17beta-trenbolone. Arch Environ Contam Toxicol. 2006;51(2):237–243. [DOI] [PubMed] [Google Scholar]
- 34.Morthorst JE, Holbech H, Bjerregaard P. Trenbolone causes irreversible masculinization of zebrafish at environmentally relevant concentrations. Aquat Toxicol. 2010;98(4):336–343. [DOI] [PubMed] [Google Scholar]
- 35.Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio). 4th ed Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- 36.Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci USA. 2013;110(34):13904–13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parant JM, George SA, Pryor R, Wittwer CT, Yost HJ. A rapid and efficient method of genotyping zebrafish mutants. Dev Dyn. 2009;238(12):3168–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn. 2009;238(12):2975–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang R, Dodd A, Lai D, McNabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin (Shanghai). 2007;39(5):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 41.Hruscha A, Krawitz P, Rechenberg A, Heinrich V, Hecht J, Haass C, Schmid B. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development. 2013;140(24):4982–4987. [DOI] [PubMed] [Google Scholar]
- 42.Leal MC, Cardoso ER, Nóbrega RH, Batlouni SR, Bogerd J, França LR, Schulz RW. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol Reprod. 2009;81(1):177–187. [DOI] [PubMed] [Google Scholar]
- 43.Selman K, Wallace RA, Sarka A, Qi X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol. 1993;218(2):203–224. [DOI] [PubMed] [Google Scholar]
- 44.Guo Y, Cheng H, Huang X, Gao S, Yu H, Zhou R. Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochem Biophys Res Commun. 2005;330(3):950–957. [DOI] [PubMed] [Google Scholar]
- 45.Wang XG, Orban L. Anti-Müllerian hormone and 11 beta-hydroxylase show reciprocal expression to that of aromatase in the transforming gonad of zebrafish males. Dev Dyn. 2007;236(5):1329–1338. [DOI] [PubMed] [Google Scholar]
- 46.Bayley M, Junge M, Baatrup E. Exposure of juvenile guppies to three antiandrogens causes demasculinization and a reduced sperm count in adult males. Aquat Toxicol. 2002;56(4):227–239. [DOI] [PubMed] [Google Scholar]
- 47.Liew WC, Orbán L. Zebrafish sex: a complicated affair. Brief Funct Genomics. 2014;13(2):172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417(6888):559–563. [DOI] [PubMed] [Google Scholar]
- 49.Yong L, Thet Z, Zhu Y. Genetic editing of the androgen receptor contributes to impaired male courtship behavior in zebrafish. J Exp Biol. 2017;220(Pt 17):3017–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berg AH, Rice CD, Rahman MS, Dong J, Thomas P. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: I. Discovery in female atlantic croaker and evidence ZIP9 mediates testosterone-induced apoptosis of ovarian follicle cells. Endocrinology. 2014;155(11):4237–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pi M, Parrill AL, Quarles LD. GPRC6A mediates the non-genomic effects of steroids. J Biol Chem. 2010;285(51):39953–39964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Natl Acad Sci USA. 2001;98(24):13728–13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor–deficient mice. Proc Natl Acad Sci USA. 2006;103(1):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinfray N, Sohm F, Caulier M, Chadili E, Piccini B, Torchy C, Porcher J-M, Guiguen Y, Brion F. Dynamic and differential expression of the gonadal aromatase during the process of sexual differentiation in a novel transgenic cyp19a1a-eGFP zebrafish line [published online ahead of print June 23, 2017]. Gen Comp Endocrinol. doi: 10.1016/j.ygcen.2017.06.014. [DOI] [PubMed]
- 55.Pang Y, Thomas P. Role of G protein–coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev Biol. 2010;342(2):194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]