Fig. 4.
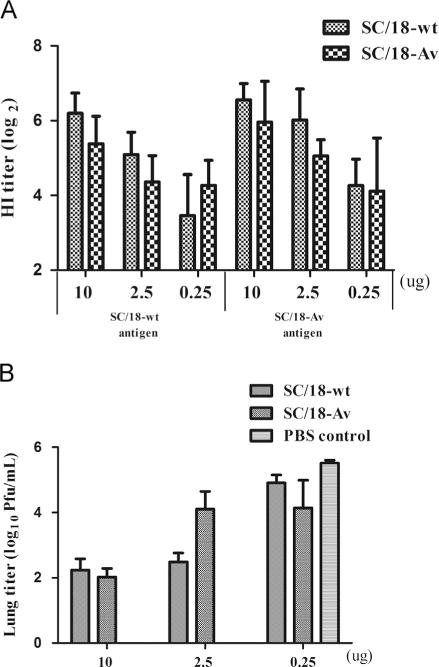
Cross-reactive antibody titers and the protective efficacy upon primary vaccination with SC/18 vaccine. (A) Groups of 10 mice are vaccinated with 100 μl of PBS-diluted inactivated whole virus vaccines at doses of 10 μg, 2.5 μg or 0.25 μg per mouse. The cross-reactive HI titers of the vaccinated mouse sera at 28 days post-primary vaccination are tested against SC/18-wt and SC/18-Av viruses. The graph represents the geometric mean value of HI antibody titers shown as log2 scale+standard deviation (SD). The HI titers between SC/18-wt and SC/18-Av at any vaccination dose are not statistically different as analyzed by two-way ANOVA and Bonferroni post-test (B). At 30 days post-primary vaccination, groups of 5 mice are challenged with 106 PFU of SC/18-wt virus and the lung titers at day 4 post-challenge are shown as the mean log10 PFU per ml+SD. There is no significance in lung titers between SC/18-wt and SC/18-Av vaccinated groups based on two-way ANOVA (p>0.05).
