Abstract
Introduction
Obstructive sleep apnea(OSA) is associated with impaired health-related quality of life(HRQL). Treatment with continuous positive airway pressure(CPAP) has variable impacts on HRQL, and this may be influenced by patient’s tolerance of therapy. The objective is to determine the impact of nocturnal supplemental oxygen(NSO) and CPAP on HRQL compared with healthy lifestyle education(HLSE) in individuals with OSA.
Methods
Patients with coronary heart disease(CHD) or at least 3 major CHD risk factors with apnea-hypopnea index of 15–50 events/hour were randomized to CPAP, NSO, or HLSE. HRQL was assessed using the Short-Form-36 (SF-36) and depression was assessed with Patient Health Questionnaire-9(PHQ-9) at baseline and 12 weeks. The treatment effect on HRQL change scores through 12 weeks was assessed using multivariable models adjusting for study site, presence of CHD at baseline, race, and baseline HRQL.
Results
A total of 318 patients were randomized to one of 3 treatment arms with 1:1:1 ratio and 94% completed baseline and follow-up HRQL instruments. Mean SF-36 scores were similar at baseline in all 3 groups ranging from 41.8±12 to 51.6±12 in various domains. In multivariable models, the CPAP group noted a significantly greater improvement than NSO in mental health (+2.33, 95% CI 0.34 to 4.31,p=0.02) and mental composite score(+2.40, 95% CI 0.40 to 4.41,p=0.02). Conversely, the CPAP group noted less improvement than NSO in physical function(−2.68, 95% CI −4.66 to −0.70,p=0.008) and physical composite score(−2.17, 95% CI −3.82 to −0.51,p=0.01). Compared to HLSE, vitality and PHQ-9 improved with CPAP but not with NSO. Significant interactions were noted between treatment effects with larger differences in black and sleepy patients.
Conclusion
These data support the use of CPAP for improving vitality, sleepiness, mental health, social functioning, and depressive symptoms in patients with OSA and established CHD or risk factors. NSO may have beneficial effects on perceived physical functioning.
Keywords: Cardiovascular disease, obstructive sleep apnea, quality of life, depression, clinical trial, outcomes
INTRODUCTION
Obstructive sleep apnea (OSA) impacts approximately 17% of women and 34% of men in the United States , and is associated with excess risk of coronary heart disease (CHD), stroke, heart failure, hypertension, and death.3–6 Patients with OSA have impaired health-related quality of life (HRQL) compared with age-matched controls and have multiple limitations due to excessive daytime sleepiness, fatigue/lack of energy, depression, and cognitive dysfunction.7–9 Cross-sectional studies are limited by the inability to determine if HRQL impairments are due to OSA severity, comorbid illnesses, or intolerance of the therapy.
Approximately 30% of patients with CHD have OSA.10 These patients may be at particularly high risk for impaired HRQL due to both CHD and OSA.11 Given the accentuated risk for both morbidity and mortality, the development of treatment strategies for improving overall health and HRQL is important. Continuous positive airway pressure (CPAP) has been the standard approach to treating moderate-to-severe OSA with a primary goal to reduce the apnea-hypopnea index (AHI).12 Meta-analyses of mostly small studies comparing CPAP to conservative therapies (or other controls) have demonstrated improvements in physical function and vitality domains, but did not demonstrate consistent improvements in other key factors affecting HRQL.13, 14 Patients treated with CPAP had more significant improvements in HRQL compared to those with sham CPAP.15 However, sham CPAP intervention may have negatively impacted HRQL, thus inflating the estimated CPAP effect on these outcomes. Furthermore, prior research has not systematically considered the effects of providing sleep hygiene and lifestyle education and support on HRQL.
Since a limitation to widespread use of CPAP has been device intolerance, there is a need to understand the value of alternative therapies on health outcomes. Limited literature has reported improvement in HRQL in patients with OSA treated with mandibular advancement devices.16 However, there has been scant research that has addressed how CPAP compares to other treatments on HRQL. Nocturnal supplemental oxygen (NSO) is commonly used by clinicians for treatment of the hypoxemia that occurs in this patient population without clear data on the impact of this therapy on outcomes.17 Limited data are available on the impact of NSO on HRQL with much of the literature focused on heart failure and central sleep apnea.18–20 Patients with underlying CHD and OSA may be at increased risk for hypoxemic-associated cardiac and cerebrovascular disease and thus NSO might improve cardiac and neuropsychiatric function, leading to improved HRQL. Thus, we aimed to determine the impact of NSO and CPAP on HRQL and depressive symptoms compared with education/lifestyle in OSA patients with co-morbid CHD or risk factors.
METHODS
Study Design
The Heart Biomarker Evaluation in Apnea Treatment (HeartBEAT) Study (ClinicalTrials.gov NCT01086800) was a National Heart, Lung and Blood Institute-funded randomized clinical trial that enrolled patients with coronary heart disease or with multiple CHD risk factors and was designed to assess the effects of CPAP or NSO therapy, each delivered with healthy lifestyle and sleep hygiene education (HLSE), versus usual care plus HLSE for improvements in cardiovascular risk. The primary outcome of the trial was 24-hour mean arterial pressure and the study design and primary results have been described in detail previously.21, 22
Participants, Randomization, and Procedures
Briefly, patients age 45–75 years with established CHD or ≥3 cardiovascular risk factors who were managed longitudinally by cardiologists in ambulatory clinics were screened for OSA with the Berlin Questionnaire.23 Patients scoring a 2 or 3 on the Berlin/questionnaire underwent home sleep testing with a portable sleep monitor (Embletta Gold, Embla Systems, Broomfield, CO) to determine eligibility for randomization. All sleep studies were scored by a single certified scorer in accordance with American Academy of Sleep Medicine guidelines, identifying all apneas and hypopneas with an oxygen desaturation of ≥3%. Patients with an apnea hypopnea index (AHI) between 15–50 events/hour were eligible for randomization after excluding those with severe OSA defined as AHI>50 events/hour or oxygen saturation <85% for >10% of the study. Patients with predominant central sleep apnea, severe daytime sleepiness (Epworth Sleepiness Scale [ESS] ≥16 or drowsy driving), or heart failure were also excluded.
Using a stratified permuted block design, patients were randomized to a) HLSE alone, b) HLSE with CPAP, or c) HLSE with NSO. The HLSE included a standardized set of educational materials (print and digital) on healthy lifestyle and sleep hygiene and strategies (including diet and exercise) to reduce cardiovascular risk modeled after American Heart Association guidelines. Patients in the CPAP arm were additionally provided an auto-titrating CPAP device (Autopap REMstar, Philips-Respironics, Inc, Murrysville, PA) that was set at a pressure range of 4–20 cm H2O for 7 days and then changed to a fixed pressure at the 90th percentile pressure required during auto-titration. Patients in the supplemental oxygen arm received nightly treatment with oxygen at 2 liters/min via nasal cannula using a stationary oxygen concentrator (EverFlo, Philips-Respironics, Murrysville, PA).
Patient Reported Outcomes
HRQL was assessed using the Short-Form-36 (SF-36),24, 25 a reliable and valid measure of generic HRQL that is commonly used in sleep apnea research.8, 26 There are eight domains, (physical function, role-physical, bodily pain, general health, energy/vitality, role-emotional, social function, and mental health), and two composite scores (mental component score [MCS] and physical component score [PCS]). The pre-specified targeted SF-36 domain for HeartBEAT was vitality (4 questions) with support from other domains, including physical functioning (10 questions) based upon responsiveness of these domains in prior studies.13 Each domain ranges from 0–100 with a higher score reflecting better HRQL. Depressive symptoms were assessed with the Patient Health Questionnaire-9 (PHQ-9), a 9-item instrument with scores ranging from 0–27 with a lower score representing less depressive symptoms27 A score ≥10 signifies significant depression consistent with DSM-IV criteria28. Both the SF-36 and PHQ-9 were measured at baseline and at 12 weeks with change scores calculated.
Statistical analysis
The analyses were based on observed data only and no statistical imputation techniques were performed for missing data beyond that described for the standard coding for SF-36. All SF-36 analyses used raw scores that were corrected to a norm-based score ranging from 0–100. Differences in continuous variables and categorical variables were assessed using ANOVA and Fisher’s exact tests respectively. Differences in change scores for patients randomized to CPAP and NSO were compared to HLSE as well as CPAP vs. NSO using ANCOVA. The ANCOVA model was created to adjust for baseline patient reported outcome responses and pre-specified key covariates of interest (study site, presence of CHD at baseline, black race). Based on clinical relevance and prior literature, effect modification by four pre-specified variables [baseline excessive sleepiness (ESS ≥ 12), baseline OSA severity (AHI ≥ 30), black race, and sex] was formally tested with interaction terms in models for each of the key HRQL measures. Since the ESS was not administered at follow-up, change in sleepiness was based on a Sleepiness Summary Score(Supplemental Methods). Spearman correlation coefficients were calculated between SF-36 and PHQ-9 change scores and changes in sleep parameters. All analyses were conducted on an intention to treat basis using SAS 9.3 (SAS Institute, Inc., Cary, NC). All p-values were based on 2-sided tests, and given the correlation among outcomes, were not adjusted for multiple comparisons.
Role of the Funding Source
The authors designed the study, oversaw data collection, performed the analyses, and prepared this publication without input from or review by the sponsor (NIH) or equipment provider (Philips-Respironics).
RESULTS
A total of 318 patients were randomized, of whom 106 were randomized into each one of three arms: CPAP, NSO, and HLSE with 298 (94%) having paired SF-36 data and 300 (94%) having paired PHQ-9 data at 12 weeks. All patients received the allocated intervention. Baseline characteristics of the patients were similar across the three groups as detailed in the primary manuscript,21 other than a higher proportion of black patients in the NSO group (Supplemental Table S1). The mean ESS scores ranged from 8.1±4 in the CPAP arm to 9.7±4 in the NSO arm. Only 85 (26.7%) patients had an ESS≥12 and this was balanced between the three groups. Baseline mean sleep duration, oxygen desaturation index(ODI), time spent at less than 90% oxygen saturation (T90%), and AHI were similar in the three groups. Hypertension, diabetes, and CHD were also well-balanced.
The three groups had similar baseline SF-36 scores (Table 1). Mean vitality scores ranged from 47.3±10 to 48.5±10 and mean physical function scores ranged from 42.0±11 to 45.1±10. Over all groups, physical composite scores (range 41.6–44.7) were lower than mental composite scores (range 48.9–51.4). Less than 20% of the cohort had depressive symptoms (PHQ-9 score≥10) with a mean score ranging from 5.2±5 to 6.3±5.
Table 1.
Baseline and 12-Week Health Status Scores Stratified by Treatment Arm
| Variable | HLSE | CPAP | NSO | |
|---|---|---|---|---|
| Vitality | ||||
| N | 100 | 99 | 101 | |
| Baseline | 48.5±10.6 | 48.2±11.4 | 47.3±10.1 | |
| 12 week | 49.5±9.4 | 51.8±11.1 | 49.8±9.0 | |
| Change from baseline | 1.0±8.4 | 3.6±9.4 | 2.5±8.3 | |
| P value | 0.2314 | 0.0002 | 0.0038 | |
| Physical Function | ||||
| N | 99 | 99 | 101 | |
| Baseline | 44.0±9.1 | 45.1±10.4 | 42.0±11.3 | |
| 12 week | 43.7±9.7 | 44.2±11.9 | 44.4±10.6 | |
| Change from baseline | −0.3±6.8 | −0.9±6.9 | 2.3±8.1 | |
| P value | 0.6246 | 0.2020 | 0.0047 | |
| MCS | ||||
| N | 99 | 99 | 100 | |
| Baseline | 48.9±12.3 | 48.9±11.0 | 51.4±12.5 | |
| 12 week | 49.7±11.0 | 52.6±10.0 | 51.9±10.1 | |
| Change from baseline | 0.8±8.1 | 3.7±8.2 | 0.5±8.8 | |
| P value | 0.3342 | <.0001 | 0.5984 | |
| PCS | ||||
| N | 99 | 99 | 100 | |
| Baseline | 43.6±9.0 | 44.7±9.5 | 41.6±9.2 | |
| 12 week | 42.9±9.3 | 44.6±10.2 | 44.1±10.5 | |
| Change from baseline | −0.8±6.4 | −0.1±5.1 | 2.4±6.2 | |
| P value | 0.2211 | 0.8275 | 0.0002 | |
| Role Physical | ||||
| N | 100 | 99 | 100 | |
| Baseline | 43.9±10.2 | 44.7±11.5 | 44.5±11.0 | |
| 12 week | 43.5±9.5 | 46.7±11.0 | 45.7±11.1 | |
| Change from baseline | −0.4±8.7 | 2.1±7.1 | 1.2±8.8 | |
| P value | 0.6284 | 0.0051 | 0.1670 | |
| Role Emotional | ||||
| N | 100 | 99 | 100 | |
| Baseline | 46.0±12.6 | 45.7±11.8 | 47.9±12.4 | |
| 12 week | 45.8±11.9 | 47.9±10.5 | 48.3±11.1 | |
| Change from baseline | −0.2±10.5 | 2.2±8.2 | 0.3±11.1 | |
| P value | 0.8540 | 0.0093 | 0.7528 | |
| Social Functioning | ||||
| N | 100 | 99 | 101 | |
| Baseline | 47.2±10.8 | 47.6±11.7 | 47.5±10.8 | |
| 12 week | 46.8±11.5 | 49.4±10.6 | 49.2±9.9 | |
| Change from baseline | −0.4±9.6 | 1.7±7.8 | 1.7±8.2 | |
| P value | 0.6916 | 0.0321 | 0.0366 | |
| Bodily Pain | ||||
| N | 100 | 99 | 101 | |
| Baseline | 46.7±10.5 | 46.8±10.2 | 44.8±8.3 | |
| 12 week | 45.7±10.6 | 47.5±11.0 | 47.0±10.0 | |
| Change from baseline | −1.1±8.8 | 0.7±7.6 | 2.3±7.6 | |
| P value | 0.2321 | 0.3500 | 0.0036 | |
| Mental Health | ||||
| N | 100 | 99 | 101 | |
| Baseline | 48.6±10.8 | 49.2±10.3 | 51.0±10.4 | |
| 12 week | 49.4±10.2 | 52.4±9.5 | 51.6±9.4 | |
| Change from baseline | 0.8±7.7 | 3.2±8.3 | 0.5±8.0 | |
| P value | 0.2729 | 0.0002 | 0.5131 | |
| General Health | ||||
| N | 100 | 99 | 101 | |
| Baseline | 43.4±9.7 | 44.9±10.9 | 43.6±8.8 | |
| 12 week | 43.3±9.3 | 46.3±9.8 | 45.2±10.0 | |
| Change from baseline | −0.1±6.1 | 1.5±6.9 | 1.6±5.8 | |
| P value | 0.8549 | 0.0391 | 0.0081 | |
| PHQ-9 | ||||
| N | 100 | 99 | 101 | |
| Baseline | 6.3±5.1 | 5.2±4.7 | 5.3±5.3 | |
| 12 week | 5.1±4.0 | 3.5±4.1 | 4.2±4.0 | |
| Change from baseline | −1.1±3.8 | −1.7±3.6 | −1.1±4.1 | |
| P value | 0.0037 | <.0001 | 0.0081 | |
| Sleepiness Summary Score | ||||
| N | 98 | 96 | 100 | |
| Baseline | 6.3±2.9 | 6.2±3.0 | 6.2±2.8 | |
| 12 week | 5.6±2.9 | 4.3±2.7 | 4.6±2.8 | |
| Change from baseline | −0.8±2.5 | −1.9±2.9 | −1.6±3.0 | |
| P value | 0.0036 | <.0001 | <.0001 | |
HLSE-Healthy lifestyle, sleep hygiene and education intervention; CPAP-Continuous positive airway pressure; NSO-Nocturnal supplemental oxygen. Categorical data represented as percentage of population and continuous data represented as mean (standard deviation); SF-36-Short Form-36; MCS-mental component score; PCS-Physical component score; PHQ-9-Patient Health Questionnaire-9. All SF-36 scores are norm-based with ranges from 0–100 with higher score representing better quality of life.
Patients randomized to CPAP and NSO reported more significant improvements in HRQL than those randomized to HLSE over 12 weeks (Table 1). Those randomized to CPAP noted an absolute increase in vitality (3.6±9) over 12 weeks while physical function was unchanged (−0.9±7) in this group. In contrast, patients randomized to NSO noted an increase in physical function (2.3±8) and vitality (2.5±8). In comparison to HLSE, there was a significant improvement in vitality over 12 weeks in patients randomized to CPAP (mean difference 2.37, p=0.028) and improvement in physical function in patients randomized to NSO (mean difference 2.35, p=0.019).
Compared with HLSE, both CPAP and NSO treated patients had statistically significantly greater improvements in social functioning and general health (Table 2). CPAP use conferred additional improvements in vitality (primary endpoint), MCS, role physical, and mental health. NSO use was associated with significant improvements in physical function (primary endpoint), PCS, and bodily pain compared with HLSE. Those treated with CPAP noted a greater improvement in PHQ-9 scores compared with HLSE (Mean difference −1.12, 95% CI: −1.97 to −0.27, p=0.010). The PHQ-9 change scores for NSO were similar to those for HLSE (p=0.30). Finally, the use of CPAP or NSO both improved self-reported sleepiness over 12 weeks in comparison to HLSE (p<0.001 and =0.016 respectively).
Table 2.
Overall 12-week Adjusted Change in Health Status Stratified by Treatment Arm
| Response | Sample size | Test if the effect of CPAP, NSO, and HLSE are the same | CPAP- HLSE | NSO - HLSE | CPAP -NSO |
|---|---|---|---|---|---|
| Mean difference in change of response (95% CI); p-value | Mean difference in change of response (95% CI); p-value | Mean difference in change of response (95% CI); p-value | |||
| MCS | HLSE: 97 CPAP: 98 NSO: 99 |
0.0102 |
2.88 (0.90, 4.87) P=0.005 |
0.48 (−1.52, 2.48) P=0.636 |
2.40 (0.40, 4.41) P=0.019 |
| PCS | HLSE: 97 CPAP: 98 NSO: 99 |
0.0011 | 0.86 (−0.77, 2.49) P=0.300 |
3.03 (1.39, 4.67) P<0.001 |
−2.17 (−3.82, −0.51) P=0.010 |
| Vitality (Primary) | HLSE: 98 CPAP: 98 NSO: 100 |
0.0865 |
2.37 (0.25, 4.49) P=0.028 |
0.89 (−1.23, 3.01) P=0.410 |
1.48 (−0.66, 3.62) P=0.175 |
| Role Physical | HLSE: 98 CPAP: 98 NSO: 99 |
0.0326 |
2.72 (0.63, 4.81) P=0.011 |
1.90 (−0.19, 4.00) P=0.075 |
0.81 (−1.30, 2.92) P=0.449 |
| Physical function (Primary) | HLSE: 97 CPAP: 98 NSO: 100 |
0.0153 | −0.34 (−2.30, 1.62) P=0.736 |
2.35 (0.38, 4.31) P=0.019 |
−2.68 (−4.66, −0.70) P=0.008 |
| Role Emotional | HLSE: 98 CPAP: 98 NSO: 99 |
0.197 | 2.11 (−0.23, 4.45) P=0.078 |
1.41 (−0.95, 3.77) P=0.240 |
0.70 (−1.68, 3.08) P=0.563 |
| Social functioning | HLSE: 98 CPAP: 98 NSO: 100 |
0.0577 |
2.30 (0.15, 4.45) P=0.036 |
2.22 (0.07, 4.38) P=0.043 |
0.08 (−2.09, 2.25) P=0.942 |
| Bodily pain | HLSE: 98 CPAP: 98 NSO: 100 |
0.0391 | 1.80 (−0.34, 3.95) P=0.099 |
2.75 (0.60, 4.91) P=0.012 |
−0.95 (−3.12, 1.22) P=0.389 |
| Mental health | HLSE: 98 CPAP: 98 NSO: 100 |
0.0138 |
2.73 (0.77, 4.70) P=0.006 |
0.41 (−1.56, 2.38) P=0.684 |
2.33 (0.34, 4.31) P=0.022 |
| General health | HLSE: 98 CPAP: 98 NSO: 100 |
0.0396 |
1.94 (0.30, 3.58) P=0.021 |
1.72 (0.08, 3.36) P=0.040 |
0.22 (−1.44, 1.87) P=0.795 |
| PHQ-9 | HLSE: 98 CPAP: 98 NSO: 100 |
0.0346 |
−1.12 (−1.97, −0.27) P=0.010 |
−0.45 (−1.30, 0.40) P=0.299 |
−0.67 (−1.52, 0.18) P=0.124 |
| Sleepiness Summary Score | HLSE: 96 CPAP: 95 NSO: 99 |
0.0017 |
−1.21 (−1.89, −0.53) P=0.001 |
−0.84 (−1.51, −0.16) P=0.016 |
−0.38 (−1.06, 0.31) P=0.278 |
ANCOVA Model covariate adjusts for site, presence of CHD at baseline, black race, and baseline PRO response. Categorical data represented as percentage of population and continuous data represented as mean (standard deviation). HLSE-Healthy lifestyle, sleep hygiene and education intervention; CPAP-Continuous positive airway pressure; NSO-Nocturnal supplemental oxygen. SF-36-Short Form-36; MCS-mental component score; PCS-Physical component score; PHQ-9-Patient Health Questionnaire-9. All SF-36 scores are norm-based with ranges from 0–100 with higher score representing better quality of life.
When comparing CPAP to NSO, those in the CPAP group reported significantly greater improvements in MCS and mental health (p=0.019 and 0.022 respectively). Non-significant trends towards improved vitality (Mean difference:1.48,p=0.175) and PHQ-9 (mean difference:−0.67,p=0.124) change scores in the CPAP compared to NSO arms were noted. Patients treated with NSO reported significantly greater improvements in PCS and physical function than did patients treated with CPAP (p=0.010 and p=0.008 respectively).
Several pre-specified subgroup analyses were performed (Figures 1 and 2). There was no interaction between treatment arm and AHI severity or sex of the patient and in change scores for SF-36 and PHQ-9 (Supplemental Table S2). However, the treatment effect on HRQL differed by the degree of baseline sleepiness and race. Larger relative improvements in MCS, vitality and depression with CPAP were generally observed among those with higher ESS scores. Similarly, compared to HLSE, NSO was associated with greater improvement in MCS and vitality among sleepier patients. Black patients also tended to have higher relative improvements with CPAP in MCS and vitality but less improvement in PCS. Finally, ODI and T90% improved with CPAP and NSO, but not with HLSE (Supplemental Table S3). However, there were not significant correlations between SF-36/PHQ-9 scores and changes in these parameters (Supplemental Table S4).
Figure 1.
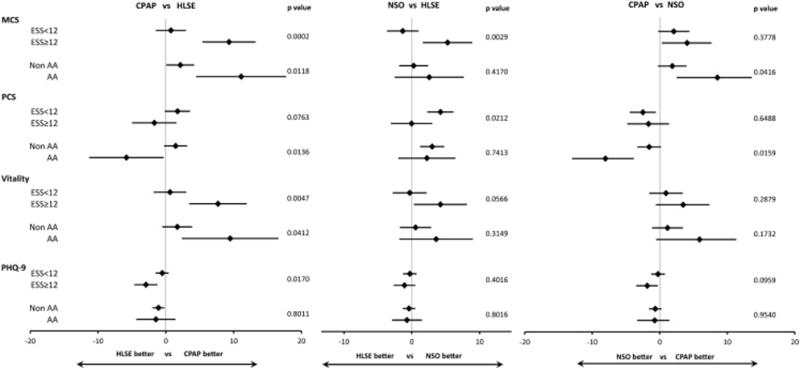
Figure 2.
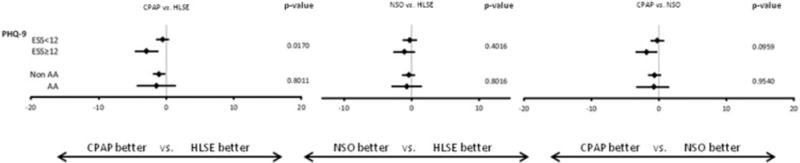
DISCUSSION
Despite the high prevalence of OSA in CHD patients and increasing evidence that OSA is associated with worse outcomes, the use of CPAP devices has been limited. Some patients with documented OSA use oxygen as an alternative to CPAP therapy often due to intolerance of standard therapy. Little data have been available regarding the impact of these therapies comparatively on HRQL, an important target of OSA interventions. In this randomized controlled study, we observed that overall HRQL is quite impaired and equal to that of patients with obesity, diabetes, chronic kidney disease,29 heart failure,30 and cancer,31 illustrating the need to improve these outcomes.32 These results are timely, as HRQL in OSA has been recently recognized as a priority area of investigation and as a key, fundamental outcome marker to examine in evaluating OSA treatment effect.33 Moreover, with the potential risk of adaptive servo-ventilation in a recent heart failure-reduced ejection fraction population, the potential benefit of oxygen therapy may be an attractive alternative for evaluation.34
Compared with HLSE alone, both CPAP and NSO were associated with improved HRQL outcomes, although their effects on physical and mental health domains and related subscores differed. NSO resulted in significantly improved physical functioning, compared to either CPAP or HLSE. In contrast, compared to HLSE, CPAP but not NSO, resulted in significant improvement in our primary outcome, vitality, as well as with the related construct of sleepiness. CPAP also was associated with an improvement in the MCS and with depressive symptoms compared to either NSO or HLSE. Both CPAP and oxygen improved social functioning and general health. Patients with more sleepiness noted greater improvements in MCS and vitality with either active treatment modality compared to HLSE alone than did the less sleepy patients. These sleepy patients also noted some improvements in depressive symptoms. We observed more improvement in MCS and vitality among black patients with CPAP therapy, but better physical functioning with NSO or HLSE.
Use of CPAP has been reported to improve several dimensions of HRQL in patients with OSA.35–40 A small study has reported significant improvements in vitality, mental health, and social functioning with short-term use of CPAP in patients with a range of sleep apnea severity treated with CPAP.41 Our results extend this literature by identifying benefit of CPAP therapy as compared to either a HLSE intervention or NSO, and by showing benefit in patients who did not seek treatment for sleep apnea but who were identified primarily through patient screening in cardiology clinics. Improvements in vitality and mental health have been attributed to improved sleep quality and cerebral oxygenation.42 The beneficial effects of CPAP therapy may be attenuated by discomfort of the mask or machine, or by negative cognitive perceptions regarding CPAP use, both of which also may contribute to non-adherence and decreased physical functioning.43, 44 Nevertheless, the current data support the use of CPAP as a primary modality for improving vitality, sleepiness, mental health, social functioning, and depressive symptoms in patients with OSA.
Limited studies have directly compared oxygen therapy to CPAP in sleep apnea patients. A small study demonstrated improvements in New York Heart Association functional status in patients receiving NSO compared with usual care,45 but did not improve HRQL in 51 heart failure patients with co-morbid predominant central sleep apnea.46, 47 Our study shows that in patients with CHD risk factors and/or CHD and OSA, NSO was not associated with improved vitality, mental health, or depressive symptoms, but was associated with improvements in the physical health composite score as well as the physical function and bodily pain subscores compared to a HLSE intervention. Furthermore, improvements in physical functioning were greater than that observed with CPAP treatment.
There is scant literature that has addressed physical functioning as an outcome of CPAP. In one large population study, MCS almost normalized among CPAP treated patients compared with non-OSA patients; however, PCS remained significantly lower in the population.40 However, data from this same cohort also showed a tendency for greater improvements in physical functioning in patients adherent to CPAP compared to those who were non-adherent, and in obese individuals. We observed comparable improvements in overnight oxygenation with use of CPAP or NSO. However, NSO use averaged about one hour more per night than CPAP use. It is possible that the longer period of improved oxygenation led to a greater improvement in physical functioning in the NSO group in comparison with the CPAP group. Finally, patients with COPD and pulmonary hypertension have used oxygen with improved HRQL.48
We found an interaction between the degree of sleepiness and changes in HRQL with treatment. A prior cross-sectional study has estimated that approximately 17% of the variance in vitality scores in individuals with mild to moderate sleep apnea is explained by level of sleepiness.49 Other studies have demonstrated greater CPAP adherence and improvements in blood pressure among patients with higher levels of sleepiness.50 We did not find that AHI severity influenced responsiveness to any intervention, although this could be influenced by the exclusion of patients with AHI>50. Our findings expand prior studies by demonstrating symptom burden is more important than OSA severity in predicting improvements in HRQL.41
We also observed several significant interactions between treatment and race. Black patients had larger treatment-associated improvements in the areas of MCS and vitality but had less improvement in physical functioning with CPAP than did others. Given the relatively small sample sizes for subgroup analyses, these results should be interpreted cautiously. However, other research has described differences in CPAP compliance51 and OSA-treatment associated improvements in behavior52 in black patients than in other race groups, supporting a need for further research.
The rationale for SF-36 use in HeartBEAT was the recommendation of the American Academy of Sleep Medicine Task Force on Sleep-Related Breathing Disorders in Adults suggesting that the SF-36 consistently rated several important domains lower than their norm-based counterparts.53 Since patients had disparate amounts of CHD, a generic instrument would be applicable across the spectrum of these patients. Avoidance of instruments specific for OSA also mitigated the potential responsiveness to CPAP therapy so that oxygen could be reliably compared. Several well-written review articles have illustrated the strengths and limitations of disease-specific and generic HRQL instruments.8, 26
There are several limitations that deserve mention. Long-term impact of therapy on HRQL cannot be assessed. As there was no sham CPAP or sham oxygen used, change scores may have been increased in patients who were more optimistic about impact of device-related treatments. Nonetheless, the consistency of CPAP and NSO effects on similar domains (CPAP positively influencing vitality and mental health domains and NSO positively impacting physical functioning domains) supports the specificity of each of their effects of HRQL. Not using a disease-specific OSA instrument may have impacted the ability to detect smaller changes in HRQL. However, the disease-specific SAQLI is highly correlated to SF-36 in OSA patients.44 Though the absolute effects noted in this analysis are modest, our results are likely an underestimate of the full impact of sleep apnea treatment on HRQL, as patients with severe sleep apnea and the most severe symptoms were excluded from the HeartBEAT study. The interaction between greater sleepiness and treatment impact on HRQL suggests that patients who are impacted by their OSA may gain greater benefit with treatment.
CONCLUSION
Patients with OSA and CHD who are treated with either nocturnal oxygen or CPAP noted improvements in HRQL. In direct comparison of the two modalities, CPAP use improved vitality and mental status domains while oxygen improved physical status domains. HRQL improvements were greater among individuals with the higher levels of sleepiness.
Supplementary Material
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute (RC2 HL101417, 1R01HL109493, and R21HL108226) and by a grant from the National Center for Research Resources (UL1 RR024989).
Dr. Lewis reports receiving grant funding for research from Resmed.
Dr. Bhatt reports grants from NIH, during the conduct of the study; grants from Amarin, grants from AstraZeneca, grants from Bristol-Myers Squibb, grants from Eisai, grants from Ethicon, grants from Medtronic, grants from sanofi aventis, grants from The Medicines Company, other from FlowCo, other from PLx Pharma, other from Takeda, personal fees from Duke Clinical Research Institute, personal fees from Mayo Clinic, personal fees from Population Health Research Institute, personal fees from American College of Cardiology, personal fees from Belvoir Publications, personal fees from Duke Clinical Research Institute, personal fees from Population Health Research Institute, personal fees from Slack Publications, personal fees from WebMD, personal fees from Elsevier Practice Update Cardiology, other from Medscape Cardiology, other from Regado Biosciences, other from Boston VA Research Institute, personal fees and non-financial support from Society of Cardiovascular Patient Care, non-financial support from American Heart Association Get With The Guidelines Steering Committee, personal fees from HMP Communications, grants from Roche, personal fees from Harvard Clinical Research Institute, personal fees from Harvard Clinical Research Institute, other from Clinical Cardiology, personal fees from Journal of the American College of Cardiology, other from VA Cardiovascular Assessment, Reporting and Tracking System (CART) Program, Research and Publications Committee, grants from Pfizer, grants from Forest Laboratories, grants from Ischemix, other from St. Jude Medical, non-financial support from American College of Cardiology, other from Biotronik, outside the submitted work.
Dr. Patel reports grants from ResMed Foundation, non-financial support from Philips Respironics, grants from American Sleep Medicine Foundation, personal fees from Apnicure, personal fees from Apnex Medical.
Dr. Quan reports grants from National Institutes of Health during the conduct of the study; personal fees from Global Corporate Challenge, personal fees from American Board of Internal Medicine, other from American Academy of Sleep Medicine, and Associate Editor, Southwest Journal of Pulmonary and Critical Care.
Dr. Mehra has received funding from the National Institutes of Health for Research (NIH NHLBI 1R01HL109493 and R21HL108226), her institution has received positive airway devices from Philips Respironics for research for which she is the Principal Investigator and has received honoraria from the American Academy of Sleep Medicine.
Dr. Redline reports grants and other from Resmed Foundation and ResMed Inc., other from Philips Respironics, from null, outside the submitted work; and Dr Redline is the incumbent of an endowed professorship donated to the Harvard Medical School by Dr. Peter Farrell, the founder and Board Chairman of ResMed, through a charitable remainder trust instrument, with annual support equivalent to the endowment payout provided to the Harvard Medical School during Dr. Farrell’s lifetime by the ResMed Company through an irrevocable gift agreement.
Dr. Punjabi reports grants and non-financial equipment support from Resmed and Respironics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
RCT# NCT01086800
Disclosures:
The following co-authors have nothing to disclose (Blumenthal, Rueschman, Wang, Weng).
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nunez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. American journal of respiratory and critical care medicine. 2014;189(12):1544–50. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumitrascu R, Tiede H, Eckermann J, et al. Sleep apnea in precapillary pulmonary hypertension. Sleep medicine. 2013;14(3):247–51. doi: 10.1016/j.sleep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the busselton health study cohort. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2014;10(4):355–62. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pamidi S, Knutson KL, Ghods F, Mokhlesi B. Depressive symptoms and obesity as predictors of sleepiness and quality of life in patients with REM-related obstructive sleep apnea: cross-sectional analysis of a large clinical population. Sleep medicine. 2011;12(9):827–31. doi: 10.1016/j.sleep.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep medicine reviews. 2003;7(4):335–49. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109(3):659–63. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 11.Lalonde L, Clarke AE, Joseph L, Mackenzie T, Grover SA, Canadian Collaborative Cardiac Assessment G Health-related quality of life with coronary heart disease prevention and treatment. J Clin Epidemiol. 2001;54(10):1011–8. doi: 10.1016/s0895-4356(01)00361-4. [DOI] [PubMed] [Google Scholar]
- 12.Leech JA, Onal E, Lopata M. Nasal CPAP continues to improve sleep-disordered breathing and daytime oxygenation over long-term follow-up of occlusive sleep apnea syndrome. Chest. 1992;102(6):1651–5. doi: 10.1378/chest.102.6.1651. [DOI] [PubMed] [Google Scholar]
- 13.Jing J, Huang T, Cui W, Shen H. Effect on quality of life of continuous positive airway pressure in patients with obstructive sleep apnea syndrome: a meta-analysis. Lung. 2008;186(3):131–44. doi: 10.1007/s00408-008-9079-5. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Xiao L, Hu J. The comparison of CPAP and oral appliances in treatment of patients with OSA: a systematic review and meta-analysis. Respiratory care. 2013;58(7):1184–95. doi: 10.4187/respcare.02245. [DOI] [PubMed] [Google Scholar]
- 15.Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. American journal of respiratory and critical care medicine. 2012;186(7):677–83. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. American journal of respiratory and critical care medicine. 2013;187(8):879–87. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 17.Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep. 2006;29(4):564–71. doi: 10.1093/sleep/29.4.564. [DOI] [PubMed] [Google Scholar]
- 18.Staniforth AD, Kinnear WJ, Starling R, Hetmanski DJ, Cowley AJ. Effect of oxygen on sleep quality, cognitive function and sympathetic activity in patients with chronic heart failure and Cheyne-Stokes respiration. Eur Heart J. 1998;19(6):922–8. doi: 10.1053/euhj.1997.0861. [DOI] [PubMed] [Google Scholar]
- 19.Javaheri S, Ahmed M, Parker TJ, Brown CR. Effects of nasal O2 on sleep-related disordered breathing in ambulatory patients with stable heart failure. Sleep. 1999;22(8):1101–6. doi: 10.1093/sleep/22.8.1101. [DOI] [PubMed] [Google Scholar]
- 20.Sasayama S, Izumi T, Seino Y, Ueshima K, Asanoi H, Group C-HS Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circulation journal: official journal of the Japanese Circulation Society. 2006;70(1):1–7. doi: 10.1253/circj.70.1. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276–85. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seif F, Patel SR, Walia HK, et al. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J Hypertens. 2014;32(2):267–75. doi: 10.1097/HJH.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 25.Bowling A, Bond M, Jenkinson C, Lamping DL. Short Form 36 (SF-36) Health Survey questionnaire: which normative data should be used? Comparisons between the norms provided by the Omnibus Survey in Britain, the Health Survey for England and the Oxford Healthy Life Survey. Journal of public health medicine. 1999;21(3):255–70. doi: 10.1093/pubmed/21.3.255. [DOI] [PubMed] [Google Scholar]
- 26.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep medicine. 2001;2(6):477–91. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 27.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. Jama. 1999;282(18):1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis EF, Pfeffer MA, Feng A, et al. Darbepoetin alfa impact on health status in diabetes patients with kidney disease: a randomized trial. Clin J Am Soc Nephrol. 2011;6(4):845–55. doi: 10.2215/CJN.06450710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry C, McMurray J. A review of quality-of-life evaluations in patients with congestive heart failure. Pharmacoeconomics. 1999;16(3):247–71. doi: 10.2165/00019053-199916030-00003. [DOI] [PubMed] [Google Scholar]
- 31.Buijs C, Rodenhuis S, Seynaeve CM, et al. Prospective study of long-term impact of adjuvant high-dose and conventional-dose chemotherapy on health-related quality of life. J Clin Oncol. 2007;25(34):5403–9. doi: 10.1200/JCO.2007.11.2813. [DOI] [PubMed] [Google Scholar]
- 32.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2015;11(3):357–83. doi: 10.5664/jcsm.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med. 2015;373(12):1095–105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkinson C, Stradling J, Petersen S. How should we evaluate health status? A comparison of three methods in patients presenting with obstructive sleep apnoea. Qual Life Res. 1998;7(2):95–100. doi: 10.1023/a:1008845123907. [DOI] [PubMed] [Google Scholar]
- 36.Piccirillo JF, Gates GA, White DL, Schectman KB. Obstructive sleep apnea treatment outcomes pilot study. Otolaryngology—head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1998;118(6):833–44. doi: 10.1016/S0194-5998(98)70277-3. [DOI] [PubMed] [Google Scholar]
- 37.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. American journal of respiratory and critical care medicine. 1999;159(2):461–7. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 38.Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. Journal of sleep research. 1997;6(3):199–204. doi: 10.1046/j.1365-2869.1997.00043.x. [DOI] [PubMed] [Google Scholar]
- 39.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. American journal of respiratory and critical care medicine. 1998;157(3 Pt 1):858–65. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 40.Bjornsdottir E, Keenan BT, Eysteinsdottir B, et al. Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. Journal of sleep research. 2014 doi: 10.1111/jsr.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure—a prospective study. Chest. 1999;115(1):123–9. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- 42.Verstraeten E, Cluydts R. Executive control of attention in sleep apnea patients: theoretical concepts and methodological considerations. Sleep medicine reviews. 2004;8(4):257–67. doi: 10.1016/j.smrv.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Sawyer AM, Canamucio A, Moriarty H, Weaver TE, Richards KC, Kuna ST. Do cognitive perceptions influence CPAP use? Patient Educ Couns. 2011;85(1):85–91. doi: 10.1016/j.pec.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. American journal of respiratory and critical care medicine. 1998;158(2):494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 45.Sasayama S, Izumi T, Matsuzaki M, et al. Improvement of quality of life with nocturnal oxygen therapy in heart failure patients with central sleep apnea. Circulation journal: official journal of the Japanese Circulation Society. 2009;73(7):1255–62. doi: 10.1253/circj.cj-08-1210. [DOI] [PubMed] [Google Scholar]
- 46.Bordier P, Orazio S, Hofmann P, Robert F, Bourenane G. Short- and long-term effects of nocturnal oxygen therapy on sleep apnea in chronic heart failure. Sleep & breathing = Schlaf & Atmung. 2014 doi: 10.1007/s11325-014-0982-0. [DOI] [PubMed] [Google Scholar]
- 47.Brostrom A, Hubbert L, Jakobsson P, Johansson P, Fridlund B, Dahlstrom U. Effects of long-term nocturnal oxygen treatment in patients with severe heart failure. J Cardiovasc Nurs. 2005;20(6):385–96. doi: 10.1097/00005082-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Eaton T, Garrett JE, Young P, et al. Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled study. The European respiratory journal. 2002;20(2):306–12. doi: 10.1183/09031936.02.00301002. [DOI] [PubMed] [Google Scholar]
- 49.Briones B, Adams N, Strauss M, et al. Relationship between sleepiness and general health status. Sleep. 1996;19(7):583–8. doi: 10.1093/sleep/19.7.583. [DOI] [PubMed] [Google Scholar]
- 50.Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respiratory care. 2013;58(9):1467–73. doi: 10.4187/respcare.02139. [DOI] [PubMed] [Google Scholar]
- 51.Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34(12):1653–8. doi: 10.5665/sleep.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–89. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


