Abstract
Background
Small-bowel capsule endoscopy (CE) is a prime modality for evaluation of the small bowel. The Lewis score (LS) and the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI) are validated endoscopic indices for quantification of small-bowel inflammation on CE. It is unclear whether these indexes are interchangeable for the evaluation of mucosal inflammation in established Crohn’s disease (CD). The aim of this study was to compare the quantitative evaluation of small- bowel inflammation by LS and CECDAI.
Methods
Patients with known quiescent small-bowel CD for at least 3 months (Crohn’s disease activity index < 150) were prospectively recruited and underwent CE. The LS was calculated using RAPID 8 capsule-reading software and the CECDAI was calculated manually. Cumulative LS (C-LS) was calculated by summation of individual tertile LS. Fecal calprotectin (FCP) and C-reactive protein (CRP) levels were measured and correlated with the scores.
Results
A total of 50 patients were included in the study. There was a moderate correlation between the worst segment LS and CECDAI (Pearson’s r = 0.66, p = 0.001), and a strong correlation between C-LS and CECDAI (r = 0.81, p = 0.0001). CECDAI < 5.4 corresponded to mucosal healing (LS < 135), while CECDAI > 9.2 corresponded to moderate-to-severe inflammation (LS ⩾ 790). There was a moderate correlation between capsule scores and FCP levels (r = 0.39, p = 0.002 for LS, r = 0.48, p = 0.001 for C-LS, and r = 0.53, p = 0.001 for CECDAI, respectively). CRP levels were not significantly correlated with either score.
Conclusions
CECDAI and C-LS are strongly correlated and perform similarly for quantitative assessment of mucosal inflammation in established CD.
Keywords: capsule endoscopy, fecal calprotectin, Crohn’s disease
Introduction
Capsule endoscopy (CE) is the modality of choice for evaluation of the entire small bowel. For diagnosis of Crohn’s disease (CD), the diagnostic yield of CE is higher than that of ileocolonoscopy and CT enterography, and overall similar to that of magnetic resonance enterography (MRE),1 though recent studies have shown its superiority over MRE in the proximal small bowel.2–4 CE is more sensitive than other modalities for detection of proximal small disease and subtle mucosal inflammation,2,3,5,6 and is more acceptable to patients in comparison to MRE.7 CE can detect active mucosal inflammation in at least 50% of patients with small-bowel CD in clinical remission.6
Currently two quantitative endoscopic inflammatory indices are available for the diagnosis and monitoring of CD. The Lewis score (LS)8 truncates the small bowel into three tertiles (by small bowel transit time) and assigns points to pathological findings (i.e. mucosal edema, ulcers, and strictures) characteristic for CD. Both severity and extent impact the scoring of the findings. The LS is incorporated in the RAPID reading software for the PillCam SB capsule (Medtronic, Dublin, Ireland). A score < 135 indicates normal or clinically insignificant mucosal inflammatory changes, 135–790 indicates mild inflammation, and a score ⩾ 790 indicates moderate-to-severe inflammation. The LS was recently validated for monitoring established CD.9,10 An additional score known as the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI) is utilized for the scoring of small- bowel inflammation on CE.11 This score divides the small bowel into two segments and includes degree and extent of mucosal inflammation and presence of strictures.10 In a previous study that included a mixed cohort of patients evaluated for suspected CD, a strong correlation between the indices (r = 0.68) was demonstrated; the correlation of LS with fecal calprotectin (FCP) was somewhat stronger than that of CECDAI.12 However, the correlation of both indices in the follow up of established CD, as well as the correlation with inflammatory biomarkers in this setting, has not been previously evaluated.
Therefore, the aim of the current study was to evaluate the correlation between LS and CECDAI, and the correlation of both indices with inflammatory biomarkers for the evaluation of established CD.
Methods
Patient population
The current study was a retrospective analysis of prospectively collected data, as part of a larger project aimed to identify predictors of clinical relapse in patients with quiescent CD.6 The study population included adult (> 18 years) patients with CD with established small-bowel disease in clinical remission or mild disease (Crohn’s disease activity index < 250) that were in corticosteroid-free remission for 3–24 months and treated with a stable medication dose (60 days for thiopurines and methotrexate and infliximab, 30 days for adalimumab and 5-aminosalicylic acid agents). All patients signed an informed consent and the study was approved by the institutional ethics review board.
CE studies
In patients with isolated small-bowel CD, an SB-III capsule (Medtronic) was ingested. In patients with established ileocolonic CD, a colonic capsule (PillCam 2 colonic capsule, Medtronic) was used. A patency capsule (PC) test was administered to all patients with active small-bowel disease detected on MRE. If a PC was not eliminated from the small bowel within 30 h, the patient was withdrawn from the study. All images were reviewed using the RAPID 8 software (Given Imaging, Dublin, Ireland). Mucosal inflammation was quantified using the LS.8 When a colonic capsule was used, the small-bowel data were reviewed and analyzed manually using the LS protocol, while for the SB III capsule it was calculated using the built-in calculator in the Rapid 8 software.8 Mucosal healing was defined as LS < 135, mild-to-moderate inflammation as LS 135–790, and moderate-to-severe inflammation as LS > 790.8 In addition to the traditional LS (derived from the score achieved by the most involved tertile), we calculated the cumulative LS (C-LS) by summation of the individual tertile scores. Proximal small bowel was defined as the first or second tertiles in accordance with small bowel transit times. CECDAI was calculated manually using the previously published algorithm11 by a different reviewer blinded to the LS results.
Inflammatory biomarkers
FCP levels were measured using the Quantum blue calprotectin kit (BÜHLMANN Laboratories AG, Basel, Switzerland). The reported value range is 30–300 μg/g.
Statistical analysis
Descriptive statistics were presented as means ± standard deviations for continuous variables and percentages for categorical variables. Categorical variables were analyzed by chi square test/Fisher’s exact test and continuous variables by the Student’s t test/Mann–Whitney U test as appropriate. We performed a Pearson correlation analysis for correlation of both total and segmental CE and MRE scores with each other and with FCP levels. Correlation r values < 0.3 were considered as a weak-to-low correlation, 0.3–0.49 as low-to-moderate, 0.5–0.69 as moderate, and ⩾ 0.7 as a strong correlation [Fleiss, 1981].13 A two-tailed p value < 0.05 was considered statistically significant. The analysis was performed using IBM SPSS statistic (Version 20.0) (Armonk, NY, USA).
Results
A total of 50 patients who underwent CE were included in the current analysis; 47 patients (94%) were in clinical remission and the rest had a mildly active disease. The clinical and demographic characteristics of the included patients are detailed in Table 1. FCP or C-reactive protein (CRP) levels were normal in 22 (44%) patients. Of the patients included in the study, 5 (10%) were in small-bowel mucosal healing, 35 (70%) had mild disease, and 15 (30%) had moderate-to-severe disease in the small bowel.
Table 1.
The characteristics of patients included in the study.
| n | % | ||
|---|---|---|---|
| Male/female | 28/22 | 56/44 | |
| Age at diagnosis (years) | 26 ± 11 | ||
| Disease duration (years) | 6 ± 5 | ||
| Disease location | Small bowel | 31 | 62 |
| Small bowel and colon | 19 | 38 | |
| Disease phenotype | Nonstructuring, nonpenetrating | 32 | 64 |
| Stricturing | 11 | 22 | |
| Penetrating | 7 | 14 | |
| Smoking status | Current | 11 | 22 |
| Never smoked | 33 | 66 | |
| Past smoking | 6 | 12 | |
| Previous surgery | 9 | 18 | |
| Perianal disease | 13 | 26 | |
| Current treatment | 42 | 84 | |
| None | 9 | 18 | |
| Thiopurine | 17 | 34 | |
| Anti-tumor necrosis factor | 13 | 26% | |
| Combined anti-tumor necrosis factor + thiopurine | 6 | 12% | |
Correlation between LS and CECDAI
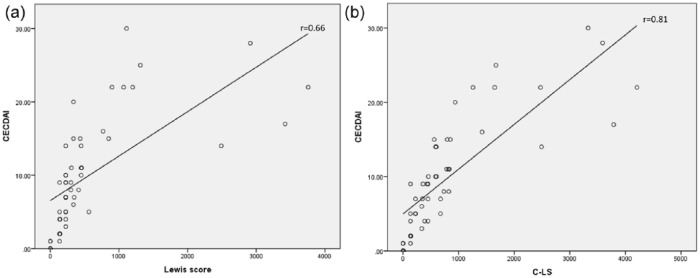
There was a moderate worst segment LS and CECDAI (Pearson’s r = 0.66, p = 0.001) and strong correlation between C-LS and CECDAI (t = 0.81, p = 0.0001) (Figure 1) for the entire small bowel. We also evaluated the correlation between segmental LS and CECDAI. Both in the proximal (first tertile for the LS and first half for the CECDAI) and the distal segment (third tertile/second half) the correlation between the scores was moderate (r = 0.53, p = 0.001 and r = 0.63, p = 0.001 for the proximal and distal small bowel, respectively). We performed a linear regression to identify the CECDAI cut-off values that correlated to the established LS cut-offs. CECDAI < 5.4 corresponded to mucosal healing (LS < 135), while CECDAI > 9.2 corresponded to moderate-to-severe inflammation (LS ⩾ 790) by linear regression.
Figure 1.
Correlation between quantitative capsule endoscopy score. (a) Worst Lewis score and CECDAI (Capsule Endoscopy Crohn’s Disease Activity Index). (b) C-LS (cumulative Lewis score) and CECDAI.
Correlation of CE scores with inflammatory biomarkers
There was a moderate correlation between both scores and FCP levels that was somewhat stronger for cumulative scores (CCECDAI and C-LS) (r = 0.39, p = 0.002 for worst LS, r = 0.48, p = 0.001 for C-LS, and r = 0.53, p = 0.001 for CECDAI). CRP levels were not significantly correlated with either score (for LS r = 0.27, p = 0.07, for C-LS r = 0.24, p = 0.09, for CECDAI r = 0.14, p = 0.33).
Discussion
Our study demonstrated a significant correlation between the two quantitative inflammation scores on CE for monitoring inflammation in established CD. Both scores have been available for over 10 years, however it was not clear whether one is superior to the other. Both LS and CECDAI incorporate similar parameters (i.e. mucosal appearance, ulcerations, and strictures) and address both the severity and extent of the findings.8,14 While LS is derived from the most severely involved segment of the small bowel (of the three tertiles divided by small bowel transit time), CECDAI is a cumulative score that represents the summation of segmental scores for the proximal and distal small bowel. Not surprisingly therefore, when LS is calculated as a cumulative score (C-LS) that summarizes the tertile scores, the correlation between the two substantially improved.
FCP is an accurate surrogate of the mucosal inflammatory burden in inflammatory bowel disease (IBD). In the recent meta-analysis of 19 studies, the pooled sensitivity and specificity of FCP for detection of active inflammation in IBD were 88% and 73%, respectively.15 Although it is currently unclear whether the accuracy of FCP is similar in the small bowel and the colon, the accuracy of FCP for detection of small-bowel inflammation as demonstrated by CE is well established, with a negative predictive value of > 90%.16 The correlation of both the LS and CECDAI with FCP is moderate, as previously reported.12 However, the correlation is improved when LS is calculated as a cumulative score; this is not surprising as a cumulative score is more likely to represent the inflammatory burden in the entire small bowel and not just a given segment.
Mucosal healing is a major therapeutic goal in CD, and is associated with a higher rate of long-term remission and lower risk of complications.17 However, clinical trials in CD still address mucosal healing by accessing the inflammation in the colon and the terminal ileum. In at least 50% of patients with CD there is proximal small-bowel involvement that can be visualized only by CE,2,18 while the degree of inflammation in one segment may entirely misrepresent the other.19 To date, very few studies assessed the efficacy of medical treatment in CD for healing the mucosa of the small bowel;20–22 clearly, if CE is to become a standard tool for evaluation of mucosal healing in CD trials, there needs to be a consensus regarding the method for quantification of the inflammation. While CECDAI was designed specifically for diagnosis and monitoring of CD, no cut-off values for this score were established; on the other hand, LS was recently validated for monitoring of the small bowel in CD with the original cut-off values of < 135 for mucosal healing. The main current advantage of LS is that it is incorporated in the viewing software of the PillCam capsules. This is the main advantage of LS, however it appears that both can be used interchangeably. The C-LS may potentially be somewhat more reflective of the small-bowel inflammatory burden than the traditional LS, however it should be validated in further studies.
Recently, a new small bowel colon capsule has been introduced in Europe, that is, the PillCam Crohn’s (Medtronic). This capsule will allow for complete pan-enteric evaluation of the digestive tract with a single diagnostic modality.23 This capsule is a potentially ideal tool for repeated monitoring in CD, in both clinical practice and in future clinical trials. The software bundled with this capsule will incorporate both the LS and the novel quantification system for both small-bowel and colonic inflammation.
In summary, our study demonstrates that both LS and CECDAI are equally suitable for quantitative monitoring of small-bowel inflammation in CD.
Acknowledgments
The author contributions were as follows: DYand UK: study design, data collection and analysis, and manuscript drafting; AL, SN, NL, and BA: data collection, and review of the manuscript for important scientific content; SB-H: study conception, initiation and design, and review of the manuscript for important scientific content; RE: study conception, initiation and design, reading of the capsule images, and review of the manuscript for important scientific content. All authors reviewed and approved the final version of the manuscript
Footnotes
Funding: The study was partially supported by a generous grant from the Leona M. and Harry B. Helmsley Charitable Trust.
Conflict of interest statement: UK received research support from Takeda Pharmaceutical Company and Janssen Pharmaceutica, speaker fees from Jannsen Pharmaceutica, AbbVie, and Takeda Pharmaceutical Company, and consultancy fees from Jannsen Pharmaceutica, Takeda Pharmaceutical Company, and CTS Pharma.
SB-H received research support and/or consultancy fees from MSD, AbbVie, Janssen Pharmaceutica, CellTrion Healthcare, and Takeda Pharmaceutical Company. RE received advisory board/lecture fee from Medtronic/Given Imaging. All the other authors declare no conflicts of interest in preparing this article.
ORCID iD: Uri Kopylov  https://orcid.org/0000-0002-7156-0588
https://orcid.org/0000-0002-7156-0588
Contributor Information
Doron Yablecovitch, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel, and Sackler School of Medicine, Tel Aviv University, Israel.
Adi Lahat, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel, and Sackler School of Medicine, Tel Aviv University, Israel.
Sandra Neuman, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel, and Sackler School of Medicine, Tel Aviv University, Israel.
Nina Levhar, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel, and Sackler School of Medicine, Tel Aviv University, Israel.
Benjamin Avidan, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel, and Sackler School of Medicine, Tel Aviv University, Israel.
Shomron Ben-Horin, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel, and Sackler School of Medicine, Tel Aviv University, Israel.
Rami Eliakim, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel, and Sackler School of Medicine, Tel Aviv University, Israel.
Uri Kopylov, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, 52621, Israel.
References
- 1. Dionisio PM, Gurudu SR, Leighton JA, et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol 2010; 105: 1240–1248; quiz 1249. [DOI] [PubMed] [Google Scholar]
- 2. Greener T, Klang E, Yablecovitch D, et al. The impact of magnetic resonance enterography and capsule endoscopy on the re-classification of disease in patients with known Crohn’s disease: a prospective Israeli IBD research nucleus (IIRN) study. J Crohns Colitis 2016; 10: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kopylov U, Klang E, Yablecovitch D, et al. Magnetic resonance enterography versus capsule endoscopy activity indices for quantification of small bowel inflammation in Crohn’s disease. Therap Adv Gastroenterol 2016; 9: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kopylov U, Yung DE, Engel T, et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn’s disease: systematic review and meta-analysis. Dig Liver Dis 2017; 49: 854–863. [DOI] [PubMed] [Google Scholar]
- 5. Amitai MM, Ben-Horin S, Eliakim R, et al. Magnetic resonance enterography in Crohn’s disease: a guide to common imaging manifestations for the IBD physician. J Crohns Colitis 2013; 7: 603–615. [DOI] [PubMed] [Google Scholar]
- 6. Kopylov U, Yablecovitch D, Lahat A, et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am J Gastroenterol 2015; 110: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 7. Lahat A, Kopylov U, Amitai MM, et al. Magnetic resonance enterography or video capsule endoscopy – what do Crohn’s disease patients prefer? Patient Prefer Adherence 2016; 10: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gralnek IM, Defranchis R, Seidman E, et al. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008; 27: 146–154. [DOI] [PubMed] [Google Scholar]
- 9. Cotter J, Dias de, Castro F, Magalhaes J, et al. Validation of the Lewis score for the evaluation of small-bowel Crohn’s disease activity. Endoscopy 2015; 47: 330–335. [DOI] [PubMed] [Google Scholar]
- 10. Rosa B, Moreira MJ, Rebelo A, et al. Lewis score: a useful clinical tool for patients with suspected Crohn’s disease submitted to capsule endoscopy. J Crohns Colitis 2012; 6: 692–697. [DOI] [PubMed] [Google Scholar]
- 11. Niv Y, Ilani S, Levi Z, et al. Validation of the capsule endoscopy Crohn’s disease activity index (CECDAI or Niv score): a multicenter prospective study. Endoscopy 2012; 44: 21–26. [DOI] [PubMed] [Google Scholar]
- 12. Koulaouzidis A, Douglas S, Plevris JN. Lewis score correlates more closely with fecal calprotectin than capsule endoscopy Crohn’s disease activity index. Dig Dis Sci 2012; 57: 987–993. [DOI] [PubMed] [Google Scholar]
- 13. Fleiss J. Statistical methods for rates and proportions. Wiley publishing, NJ, USA: 1981. [Google Scholar]
- 14. Gal E, Geller A, Fraser G, et al. Assessment and validation of the new capsule endoscopy Crohn’s disease activity index (CECDAI). Dig Dis Sci 2008; 53: 1933–1937. [DOI] [PubMed] [Google Scholar]
- 15. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015; 110: 802–819; quiz 20. [DOI] [PubMed] [Google Scholar]
- 16. Kopylov U, Yung DE, Engel T, et al. Fecal calprotectin for the prediction of small-bowel Crohn’s disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2016; 28: 1137–1144. [DOI] [PubMed] [Google Scholar]
- 17. Shah SC, Colombel JF, Sands BE, et al. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther 2016; 43: 317–333. [DOI] [PubMed] [Google Scholar]
- 18. Flamant M, Trang C, Maillard O, et al. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis 2013; 19: 1390–1396. [DOI] [PubMed] [Google Scholar]
- 19. Carvalho PB, Rosa B, Cotter J. Mucosal healing in Crohn’s disease – are we reaching as far as possible with capsule endoscopy? J Crohns Colitis 2014; 8: 1566–1567. [DOI] [PubMed] [Google Scholar]
- 20. Niv E, Fishman S, Kachman H, et al. Sequential capsule endoscopy of the small bowel for follow-up of patients with known Crohn’s disease. J Crohns Colitis 2014; 8: 1616–1623. [DOI] [PubMed] [Google Scholar]
- 21. Hall B, Holleran G, Chin JL, et al. A prospective 52 week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. J Crohns Colitis 2014; 8: 1601–1609. [DOI] [PubMed] [Google Scholar]
- 22. Hall BJ, Holleran GE, Smith SM, et al. A prospective 12-week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. Eur J Gastroenterol Hepatol 2014; 26: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 23. Leighton JA, Helper DJ, Gralnek IM, et al. Comparing diagnostic yield of a novel pan-enteric video capsule endoscope with ileocolonoscopy in patients with active Crohn’s disease: a feasibility study. Gastrointest Endosc 2017; 85: 196–205. e1. [DOI] [PubMed] [Google Scholar]