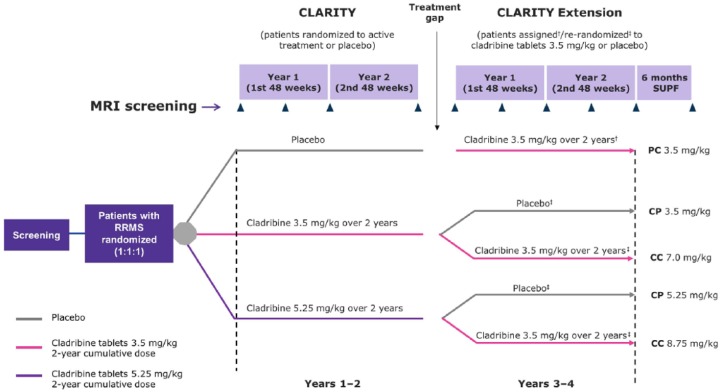
Figure 1.
CLARITY and CLARITY Extension study plan.
Assessments of MRI activity were carried out when patients entered CLARITY Extension and after Weeks 24, 48, 72 and 96 of double-blind treatment, and at the start and end of a 6-month supplemental follow-up period (unless the final double-blind period assessment was within 4 weeks of the start of the supplemental follow up).
†patients who received placebo in CLARITY were assigned to treatment with cladribine 3.5 mg/kg in CLARITY Extension.
‡patients who received cladribine 3.5 mg/kg or 5.25 mg/kg in CLARITY were rerandomized to receive placebo or cladribine 3.5 mg/kg in CLARITY Extension.
PC 3.5 mg/kg, placebo in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; CP 3.5 mg/kg, cladribine 3.5 mg/kg in CLARITY/placebo in CLARITY Extension; CC 7.0 mg/kg, cladribine 3.5 mg/kg in CLARITY/cladribine 3.5 mg/kg in CLARITY Extension; CC 8.75 mg/kg, cladribine 5.25 mg/kg in CLARITY/cladribine 3.5 mg/kg CLARITY Extension; CP 5.25 mg/kg, cladribine 5.25 mg/kg in CLARITY/placebo in CLARITY Extension; SUPF, supplemental follow up; MRI, magnetic resonance imaging; RRMS, relapsing–remitting multiple sclerosis.