Short abstract
Immunocytokines are fusion proteins that combine the specific antigen binding capacities of an antibody or derivative thereof and the potent bioactivity of a cytokine partner. These novel biopharmaceuticals have been directed to various targets of oncological as well as non-oncological origin and a handful of promising constructs are currently advancing in the clinical trial pipeline. Several factors such as the choice of a disease specific antigen, the antibody format and the modulatory nature of the payload are crucial, not only for therapeutic efficacy and safety but also for the commercial success of such a product. In this review, we provide an overview of the basic principles and obstacles in immunocytokine design with a specific focus on single chain antibody fragment-based constructs that employ interleukins as the immunoactive component.
Impact statement
Selective activation of the immune system in a variety of malignancies represents an attractive approach when existing strategies have failed to provide adequate treatment options. Immunocytokines as a novel class of bifunctional protein therapeutics have emerged recently and generated promising results in preclinical and clinical studies. In order to harness their full potential, multiple different aspects have to be taken into consideration. Several key points of these fusion constructs are discussed here and should provide an outline for the development of novel products based on an overview of selected formats.
Keywords: Immunocytokines, single-chain antibody fragment, interleukin, cancer, inflammatory diseases
Introduction
At the dawn of the 20th century, German physician and scientist Paul Ehrlich postulated the existence of specific receptors which were either cell associated or distributed in the blood stream. He proposed that these “side-chains of immunity” would respond to their specific antigens which were first thought to encompass toxins and nutrients, but were later extended to include drugs of all kinds.1 Following the replacement of the term “side-chain” with “receptor,” Ehrlich further developed his immunological theories and was awarded the Nobel Prize for Physiology/Medicine in 1908 for his achievements. Based on his experience with hundreds of different dyes for the staining of cellular structures, the concept of the “Zauberkugel,” the portentous “magic bullet”, emerged.2 According to this idea, the directed obliteration of invading parasites should be achievable by targeting receptors that are not shared with the host. This would potentially diminish the probability of causing adverse effects in patients and contribute to an improved therapeutic index. More than a hundred years later, Ehrlich’s concept has proven to be vital in the combat of infectious and non-infectious diseases alike and is more than ever exemplified by the use of monoclonal antibodies (mAb) and derivatives thereof aiming at an astonishing range of targets.3,4 In view of the relative success of antibody-drug conjugates to specifically deliver chemotherapeutic and radioactive payloads to various cancerous5 and non-cancerous6,7 maladies on one hand and the proven pharmacological efficiency of immunomodulatory proteins (cytokines) on the other, a new class of therapeutics emerged two decades ago.8 These fusion proteins were referred to as “immunocytokines” and combined the targeting moiety of mAbs or antibody fragments with the beneficial effects of pro-inflammatory (e.g. interleukin (IL)-2) or anti-inflammatory (e.g. IL-10) cytokines. As the antibody format and the choice of the “payload” can have a profound impact on the overall performance and the mode of action of the immunocytokine, this review provides an overview of existing molecular arrangements in the preclinical and clinical phase setting, with a specific focus on those that employ single chain antibody fragments (scFvs) as their targeting moiety and interleukins as the biologically active component.
The rationale of targeted cytokine delivery
Cytokines are generally present as soluble factors that can act as regulators and mediators of the innate and the adaptive immune systems but have also been found to play a role in tissue homeostasis such as in hematopoiesis. They are able to function locally as autocrine, juxtacrine or paracrine response modifiers and unfold their effects upon interaction with their specific receptors expressed in the cell membranes of their target cells. The classification of peptide signaling molecules as hormones rather than cytokines is not clear cut, since the receptors for peptide hormones and cytokines are closely related structurally9 and the sites of synthesis and action of both cytokines and some peptide hormones (e.g. growth hormone and prolactin) known to be diverse.10 Moreover, it is now known that peptide hormones operate through both paracrine and juxtacrine, as well as endocrine mechanisms. In contrast to hormones, cytokines are not secreted by cells of special glands and affect a range of biological processes such as inflammation, wound healing, organogenesis, and oncogenesis.11,12 The potential of cytokine-based immunotherapy was initially exemplified by the approval of recombinant interferon α (IFN-α) for various indications including high-risk melanoma, non-Hodgkin lymphoma (NHL), renal cell carcinoma (RCC), hairy cell leukemia or chronic viral hepatitis,11,13 followed by the introduction of IL-2 (Aldesleukin) into the clinic.14 By now, a considerable number of different interleukins (IL-7, IL-10, IL-12, IL-15, IL-21), interferons (IFN-β for multiple sclerosis, IFN-γ for chronic granulomatous disease),15 and cytokines of the tumor necrosis factor (TNF) family (TNF-α in irresectable soft tissue sarcoma and TNF-related apoptosis-inducing ligand (TRAIL) in various cancers) have been approved or are currently advancing through clinical trial pipelines.16,17 However, systemic administration of these drugs often results in dose-dependent off-target and adverse effects which may prevent dose escalation to therapeutically effective regimens in many cases. This is especially true when it comes to the treatment of malignancies that require a high local concentration of the cytokine. As they are pleiotropic in nature with functional redundancies, adverse effects of different cytokines can overlap. Examples include the induction of fever and flu-like symptoms but also more severe hematologic, endocrine, pulmonary, autoimmune, neurologic, and even psychiatric events.18 Consequently, genetic fusion of the cytokine of interest with a suitable antibody capable of targeting the site of the disease and/or the specific cells involved in pathology, whilst reducing activity in unaffected tissues, should in theory increase the therapeutic index by localizing the biological activity of the payload. Thus, a substantial broadening of the therapeutic window is expected.
Formats and characteristics of immunocytokines
A variety of different mAbs and recombinant antibody formats have been considered for immunocytokine development in the past. In principle, they can be divided into seven groups according to their structural features, comprising either (1) intact IgG molecules, (2) scFv-Fc molecules (3) small immunoproteins (minibodies) (4) the Fc fragment of an immunoglobulin, (5) mono- and bivalent antigen binding fragments (Fab and F(ab′)2), (6) scFvs, and (7) diabodies and tribodies. Compared to scFvs, diabodies and tribodies exhibit an increased valency for the targeted antigen which consequently leads to an augmented avidity. Additionally, the composition of the final product is also influenced by the cytokine itself and greatly depends on the number of monomeric units that are required to form a fully functional cytokine assembly. While many cytokines act as monomers (e.g. IFN-α, IL-2), some are only active when present as homodimers (e.g. IFN-γ, IL-22), heterodimers (e.g. IL-12 and IL-27), or homotrimers (e.g. TNF-α).19–21 This has to be taken into account when designing novel constructs as the use of a homodimeric cytokine with an scFv for example will generate a fusion protein that is bivalent for the antigen with increased overall size. By analogy, trivalent single chain derivatives have also been produced fusing a TNF-α monomer to an scFv. Homotrimeric assembly was facilitated by the cytokine moiety of the construct.22
The ultimate choice of the immunocytokine format has to be considered carefully and depends on a variety of factors. Intact IgG molecules are generally of larger size (∼150 kDa) which contributes to a longer circulatory half-life but diminishes tissue penetration.23 The primary reason for the increased circulatory persistence can be found in the presence of the Fc component involved in neonatal Fc receptor binding and recycling. Consequently, Fc domain fusions are often used to increase the half-life of cytokines, but alone do not provide targeting specificity.23 By contrast, Fc receptor binding can negatively influence in vivo half-life by increasing blood clearance as shown for an IL-2-based immunocytokine.24 However, the Fc fragment in context of an intact targeting domain can contribute to anti-tumor activity by the recruitment of antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytolysis (CDC).25 Thus, pharmacologic activity of an IgG-based immunocytokine does not only rely on the cytokine alone, but might be further modified by the immunoglobulin moiety.26 Interestingly, employing antibodies for the delivery of the cytokine does not always occur in a targeted way. Due to their size, full-length antibody constructs might face several limitations when directed against antigens located in the extravascular or perivascular space. In order to reach them efficiently, immunocytokines are required to leave the vascular environment and localize at the site of the disease.27 Apart from large size, extensive glycosylation and extreme pI values, a systemic excess of cytokine receptors or circulating binding proteins and persistent interaction with the Fcγ-receptor in other tissues might additionally prevent therapeutic efficiency of IgG-based constructs.28–30 Gillies et al.24 demonstrated that reducing the interaction of the Fc part with its receptor by mutational means contributed to a higher efficiency of an IL-2-based fusion protein. Despite these putative disadvantages in tissue penetration, intact immunoglobulins generally exhibit a better tumor uptake than smaller antibody fragments owing to the extended circulatory half-life.31
Single-chain fragments are considerably smaller and therefore display better tumor infiltration. Monomeric scFvs have a molecular weight of around 27 kDa and combine the variable domain of the light and heavy immunoglobulin chains connected by a short peptide linker.32 A single scFv is usually inferior compared to a mature IgG molecule or diabody in terms of avidity due to a monovalent single antigen binding site. This typically translates into reduced retention times at the actual tumor site (Figure 1).33,34 In an attempt to improve the retention time of scFv immunocytokines, intermediate sized constructs with two (mono- and bispecific diabodies) and even three (tribodies) single chain moieties, scFv-Fc and scFv-CH3 (minibodies) fusions as well as Fab-cytokine combinations have been considered.35 By contrast, Adams et al.36 could show that scFvs with extremely high affinity for their tumor antigen displayed impaired tumor penetration properties as they were primarily found to be retained in the perivascular regions of the tumor mass. This has to be taken into account when novel antibodies or derivatives thereof are being developed or an existing one is subjected to in vitro affinity maturation strategies.
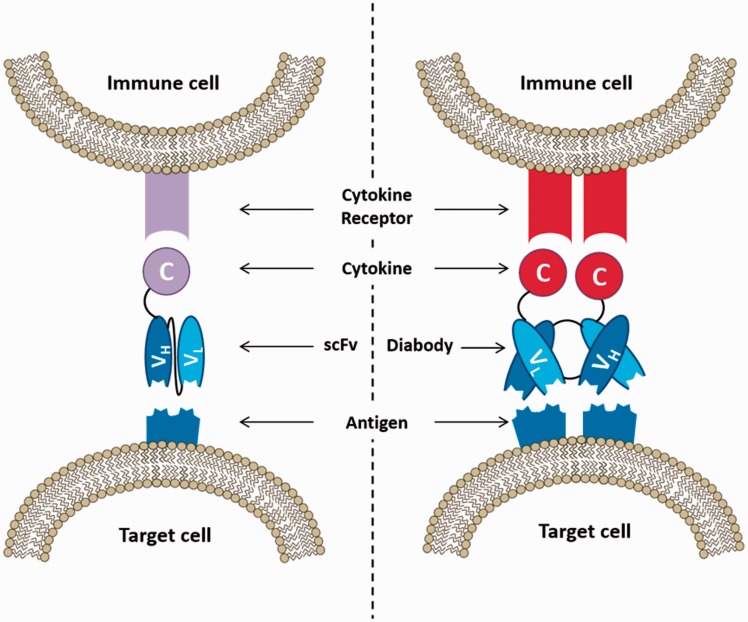
Figure 1.
Diagrammatic comparison between the mode of action of immunocytokines based on the monomeric scFv and the homodimeric diabody format. Binding of the targeting moiety to the respective antigen on the target cell enables the recruitment of immune cells via the specific cytokine receptor. Due to their increased valency, diabodies usually exhibit longer retention times at the actual target site. (A color version of this figure is available in the online journal.)
In general, tumor infiltration of macromolecular biologics is a highly complex process that involves several steps such as extravasation across tumor capillaries, diffusion, and antigen binding within the tumor interstitium, internalization, and catabolic processing by tumor cells. A detailed discussion about these processes is beyond the scope of this review but can be found in an excellent publication by Thurber et al.31 While the effect of several different formats have already been explored in animal tumor models, only IgG and scFv-based immunocytokines have progressed into clinical trials so far.37 A comprehensive overview of different antibody formats and immunocytokines that use scFvs and diabodies as targeting moiety is provided in Figure 2.
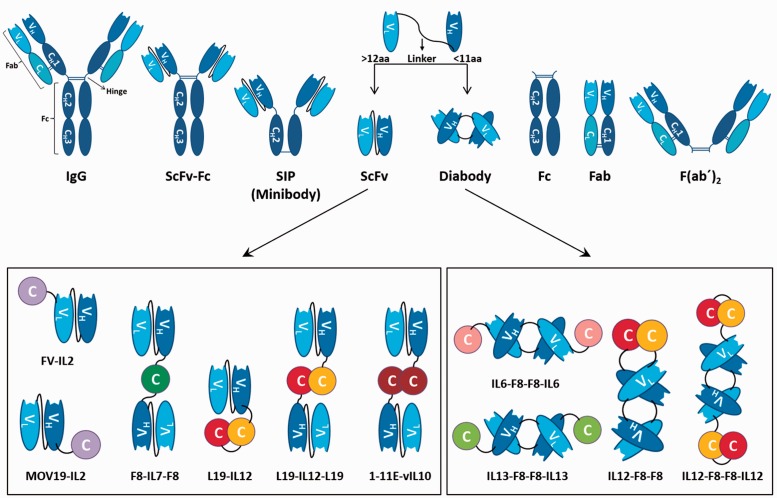
Figure 2.
Comprehensive overview of putative antibody formats and immunocytokines based on the scFv and the homodimeric diabody format. The length of the linker between the VL and the VH segment determines if a monovalent scFv (linker size >12 aa) or a divalent diabody (linker size <11 aa) is formed. Depending on the modular arrangement of the cytokine and the targeting moiety, scFv-based immunocytokines can either be monovalent (e.g. MOV19-IL2) or divalent (e.g. F8-IL7-F8) for the respective antigen. The name of a representative immunocytokine is given below every construct. (A color version of this figure is available in the online journal.)
Targeted antigens in oncological malignancies
Selection of an appropriate antigen is crucial when employing immunocytokines in oncological as well as in inflammatory or autoimmune disease applications. In view of the potential toxicities especially for potent proinflammatory cytokines, antigen targets have to be identified that are highly expressed at the site of the disease but are low or absent in normal tissues. Following this paradigm, localized enrichment of the cytokine payload via the antigen-binding moiety can be ensured.
Extensive genetic variations in tumor cells result in remodeling of the tumor proteome, including changes in post-translational modifications, and create a cell surface and secreted proteome that can differ significantly from that found in healthy cells.38 As a result, various tumor-associated cell surface antigens including the disialoganglioside GD2, the B-lymphocyte antigen CD20, epithelial cell-adhesion molecule (EpCAM), carbonic anhydrase 9 (CAIX) and fibroblast activation protein (FAP) have been targeted by both therapeutic mAbs and immunocytokines.39–43 Furthermore, extracellular matrix (ECM) proteins as part of the altered microenvironment of the tumor and nuclear structures emerging from the core of necrotic tumors have also been selected as suitable antigens for therapeutic applications. Employing in vivo biotinylation and mass spectrometry techniques, fibronectin and tenascin (TNC) splice variants were identified as promising ECM tumor markers and accessible targets.44,45 In contrast to the splice variants found in the tumor microenvironment, the glycoprotein fibronectin is highly abundant in the ECM of mammalian tissues and in the plasma. However, the aforementioned tumor remodeling events induce the insertion of two additional domains (extradomain A and B – EDA and EDB) into fibronectin which are oncofetal forms normally only expressed in fetal development.46 Consequently, EDA and EDB splice variants are ubiquitous in solid tumors but virtually undetectable in healthy individuals. Similarly, alternatively spliced isoforms of TNCs arise exclusively at the site of neo-angiogenesis in solid tumors. In particular, high expression levels of C domain containing TNC have been detected in perivascular regions of brain and lung tumors.47 By contrast, antigens released from the necrotic core of certain tumor variants comprise molecules that are present but inaccessible in cells of unaffected tissues.48 Antibodies targeting not only histones but also extracellular DNA have been described and combined with cytokines for therapeutic applications.49
Despite promising results in preclinical animal studies concerning a wide variety of cell surface, ECM and nuclear antigens, only some of them have progressed to the clinical trial phase. Notably, 5 out of 12 immunocytokines which have been assessed for their therapeutic potential are directed against splice variants of fibronectin and TNC; five fusion proteins recognize cell surface antigens (CD20, CEA, GD2, FAP and EpCAM); and two constructs detect extracellular DNA released from necrotic lesions.41,49–55
Targeted antigens in non-oncological diseases
While the antigens found in chronic inflammatory diseases or auto-immune diseases might be less defined as those that are often found in cancerous tissue, there is an opportunity to target specific differentiation antigens on the cell types critical to pathogenesis of those diseases. Moreover, inflammation per se can be regarded as a common feature shared by both maladies giving rise to a certain redundancy in antigen availability.56 Regarding cellular structures, neutrophils and macrophages appear to be promising targets as they gather at the site of inflammation in considerable numbers. Despite the fact that many of their surface antigens are additionally expressed on other circulating leukocytes, the Fcγ receptor 1 (FcγR1 – also known as CD64) has been identified as a promising target in animal models of skin inflammation, rheumatoid arthritis (RA), and ischemia-induced kidney injury.57,58 As CD64 is constitutively expressed only on macrophages, monocytes and their progenitors, off-target effects of the immunotoxin used in the aforementioned models seem to be limited. Apart from being used to treat lymphomas and leukemias, targeting the human B-cell surface antigen CD20 has proven valuable for the treatment of autoimmune diseases and chronic inflammatory disorders. As an example, the chimeric mouse–human mAb rituximab is efficacious in seropositive RA by downregulating the B-cell receptor and inducing CD-20-positive B-cell apoptosis.59 Alternatively, modified components of the ECM, particularly EDA and EDB splicing variants of fibronectin provide interesting targets in inflammatory processes as they could allow adoption of antibodies that have been developed for oncological pharmacodelivery.60 Along with the extra-domain A1 containing isoform of TNC-C, EDA and EDB are not only abundant in solid cancer forms but present a general characteristic of the neo-angiogenesis which is often found in chronic inflammatory disorders. Examples include RA, osteoarthritis (OA), chronic skin inflammation, ulcerative colitis, endometriosis, and vasculopathy.61–67 Moreover, oxidative stress has been shown to play a role in remodeling ECM components. In particular, postranslationally modified collagen type 2 (ROS-CII) has been used as an epitope to extract an scFv (1–11E) from a human antibody library that was able to bind to damaged cartilage, a major characteristic in RA and OA.68
Potential immunogenicity of antibody fusion constructs
Administration of large molecule-based biotherapeutics has a potential disadvantage compared to traditional small molecule drugs which is the ability to produce immunogenicity against the therapeutic in form of anti-drug antibodies (ADAs). The use of recombinant human cytokines coupled to humanized or fully human antibodies and antibody fragments does not necessarily guarantee central tolerance in human patients and has the capacity to prevent potentially promising molecules from being used in the clinic. This aspect becomes important if a therapeutic protein is intended to serve as long-term treatment as ADAs are usually detected within one or two weeks after the first protein administration and are typically boosted by subsequent medication cycles. Notably, a neutralizing response can not only decrease the efficacy of biotherapeutics but also elevate the risk for adverse events such as anaphylaxis, infusion reactions, or immune-complex-mediated diseases.69,70 With regards to mAbs, 84% of patients showed a positive ADA response for murine-derived antibodies which was diminished to 40% for chimeric antibodies and further reduced to 0–12% for humanized and fully human products.71 In a unique case, even a fully human therapeutic antibody such as ATR-107 (an anti-IL-21 receptor antibody) was found to elicit ADAs in a high number of healthy subjects.72 Additionally, examples of immunogenic recombinant human cytokines such as IL-2,73 INF-β,74 INF-α,75 and GM-CSF76 can be found in the literature. This seems somewhat surprising as the primary sequence of these proteins is essentially human. Nevertheless, differences in secondary, tertiary, and surface structures (i.e. glycosylation patterns) owing to heterologous expression in bacterial, mammalian or insect cell lines might be sufficient to elicit a neutralizing immune response. Furthermore, and this is particularly important in the area of immunocytokines, linker sequences may represent a target epitope for ADAs as they are designed to provide certain characteristics (e.g. flexible separation of two domains) rather than low immunogenicity. A strategy to circumvent this problem may be the selection of natural linker sequences that connect individual domains in native proteins as they are expected to exhibit lower immunogenicity then artificial sequences. By contrast, widely used flexible (Gly4Ser)n linker might also be considered as they have a low antigenicity due to lacking hydrophobic side chains needed to provide binding affinity.
When it comes to the prediction of immunogenicity of a certain biotherapeutic, animal models are of limited use as they will not be tolerant to human proteins. However, transgenic mice and xenograft transplantation models have been introduced and proven to be of some value, especially during the assessment of antibody products.77,78 Alternatively, non-human primates have been employed as a model system but also face significant limitations. Apart from ethical issues that arise from the use of these animals in preclinical trials, their MHC molecules have been found to be surprisingly different to the human equivalent. Thus, they seem less suited for the evaluation of immunogenicity than efficacy.79 Advances in computational prediction algorithms have enabled high throughput screening of peptides in silico.80 These methods provide the advantage of rapidly screening large numbers of potential candidates for their ability to bind to MHC class II molecules. However, a high level of false positives is not uncommon as not all binders eventually trigger a T-cell response.81 Consequently, T-cell culture systems can be used complementary to overcome some of the in silico limitations by providing means to directly measure antigen specific activation of T-cells.82 Recently, novel tools were developed to assess DC binding, intracellular trafficking, and activation as therapeutic proteins that efficiently interact with the antigen presentation machinery may potentially enhance immunogenicity.72
In order to reduce the immunogenicity of protein therapeutics, depletion of T-cell epitopes identified by in silico amino acid sequence analyses and in vitro T-cell-based assays has proven to be a promising strategy.83 While this approach seems to work particularly well for the deimmunization of antibodies, immunogenicity of humanized and fully human products often resides in the CDR regions.84 As these areas confer both specificity and affinity for the antigen, modifications can potentially lead to diminished bioactivity but also introduce de novo CD4+ T-cell epitopes. Careful optimization by using a combination of computational and in vitro cell-based assays is crucial to reduce immunogenicity of a product that has been shown promising bioactivity in preclinical and clinical studies but has concerns due to considerable immunogenicity.84
Single chain fragment-based immunocytokines in the preclinical and clinical trial setting
While a large number of different antibody formats and cytokines have been used to generate novel fusion constructs, the following section only focuses on those that employ monomeric scFvs and homodimeric diabodies as a targeting moiety and interleukins as the bioactive component. A comprehensive list of immunocytokines discussed in this section can be found in Table 1.
Table 1.
Comprehensive summary of immunocytokines discussed in this review.
| Name | Format (target) | Developmental stages (model/indication) | Refs |
|---|---|---|---|
| IL-1β | |||
| F8-IL1β | Diabody (EDA-FN) | Preclinical (F9) | 85 |
| IL-2 | |||
| H17E2scFv-IL2 | scFv (PLAP) | Preclinical (N.A.) | 86 |
| FV-IL2 | scFv (TAG72) | Preclinical (colorectal carcinoma) | 87 |
| FUscFv-IL2 | scFv (MK-1) | Preclinical (N.A.) | 88 |
| IL2-MOV19 | scFv (FR) | Preclinical (C26) | 89 |
| F8-IL2 | Diabody (EDA-FN) | Preclinical (NSCLC) | 90 |
| L19-IL2 | Diabody (EDB-FN) | Preclinical + gemcitabine (pancreas carcinoma)Preclinical (rheumatoid arthritis)Phase I (mRCC) Phase I + dacarbazine (metastatic melanoma) Phase I/II + rituximab (NHL) Phase II (metastatic melanoma) Phase III + L19-TNFα (metastatic melanoma) | 51,91–95 |
| F16-IL2 | Diabody (TNC-A) | Preclinical + doxorubicin/paclitaxel (breast carcinoma) Phase I (AML) phase I + BI 836858 (AML) Phase Ib + doxorubicin (solid tumors) Phase II + doxorubicin (breast carcinoma)vPhase I + cytarabine | 52,96–99 |
| IL-4 | |||
| F8-IL4 | Diabody (EDA-FN) | Preclinical (skin inflammation) Preclinical (endometriosis) Preclinical + dexamethasone (rheumatoid arthritis) Preclinical + F8-IL2 (F9, CT26, A20) Preclinical + IL12-F8-F8 (F9, CT26, A20) | 100–103 |
| IL-6 | |||
| F8-IL6 | Diabody (EDA-FN) | Preclinical (F9) | 85 |
| IL-7 | |||
| F8-IL7-F8 | scFv (EDA-FN) | Preclinical (F9) | 104 |
| IL-9 | |||
| F8-IL9 | Diabody (EDA-FN) | Preclinical (F9) | 105 |
| IL-10 | |||
| L19-IL10 | scFv (EDB-FN) | Preclinical (rheumatoid arthritis)Preclinical (skin inflammation) | 61,62 |
| F8-IL10 | Diabody (EDA-FN) | Phase I + methotrexate (rheumatoid arthritis) | 63,106 |
| 1E-11-vIL10 | scFv (ROS-CII) | Preclinical (rheumatoid arthritis) | 107 |
| IL-12 | |||
| IL12-L19 | scFv (EDB-FN) | Preclinical (metastatic lung cancer, C51, F9) | 108 |
| L19-IL12-IL19 | scFv (EDB-FN) | Preclinical (F9) | 29 |
| F8-IL12-F8 | scFv (EDA-FN) | Preclinical (F9) | 109 |
| IL12-F8-F8 | Diabody (EDA-FN) | Preclinical (F9) Preclinical + paclitaxel (F9) | 110 |
| IL12-F8-F8-IL12 | Diabody (EDA-FN) | Preclinical (F9) Preclinical + paclitaxel (F9) | 110 |
| αHER2-IL12 | scFv (HER2) | Preclinical (bladder tumor) | 111 |
| HRS3-IL12 | scFv (CD30) | Preclinical (NHL) | 112 |
| IL12-SS1 | scFv (mesothelin) | Preclinical (mesothelioma) | 113 |
| IL-13 | |||
| IL13-F8-F8-IL13 | Diabody (EDA-FN) | Preclinical (F9, Wehi-164 fibrosarcoma) | 114 |
| IL-15 | |||
| L19-IL15 | Diabody (EDB-FN) | Preclinical (F9, C51) | 115 |
| scFV-RD-IL15 | scFv (FAP) | Preclinical (B16) | 116 |
| RD-IL15-scFv-4-1BB | scFv (FAP) | Preclinical (B16) | 117 |
| IL-17 | |||
| F8-IL17-IL17-F8 | scFv (EDA-FN) | Preclinical (F9) | 118 |
| IL-22 | |||
| IL22-F8 | Diabody (EDA-FN) | preclinical (F9, ulcerative colitis) | 119 |
FN: fibronectin; F9: teratocarcinoma; PLAP: placental alkaline phosphatase; TAG: tumor associated glycoprotein; FR: folate receptor; C26: adenocarcinoma; NSCLC: non-small cell lung cancer; mRCC: metastatic renal cell carcinoma; NHL: non-Hodgkin lymphoma; TNC: tenascin; AML: acute myeloid leukemia; CT26: colon carcinoma; A20: lymphoma; ROS-CII: postranslationally modified collagen type 2; C51: adenocarcinoma; FAP: fibroblast activation protein; B16: lung metastasis.
IL-1β fusion constructs
In contrast to its membrane-bound agonist IL-1α, IL-1β is a soluble protein and can therefore act systemically. Both proteins are part of the IL-1 family whose 11 members interact with the IL-1 receptor that exists in two different forms. This is indicative of the pleiotropic nature of IL-1 which has been found to be involved in multiple biological processes such as regulation of the immune response, hematopoiesis, and inflammation. While receptor type I primarily mediates inflammatory stimuli of IL-1, type II receptor abolishes IL-1 activity together with the corresponding receptor antagonist.120 Therapeutic efficiency of recombinant human IL-1β (rhIL-1β) in patients with metastatic or unresectable solid tumors was tested in a phase I clinical trial, evaluating dose levels up to 200 ng/kg. Despite remarkable hematologic effects, extensive toxic side-effects at all tested dose levels restrict the usefulness of rhIL-1β as a systemically applied drug.121 So far, only preclinical data for an IL-1β-based immunocytokine are available. A fusion construct with the F8 antibody (a monospecific diabody) that recognizes the EDA variant of fibronectin was tested in immunocompetent mice bearing subcutaneously grafted F9 teratocarcinomas. A 10% body weight loss at a 5 μg dose and less than 50% inhibition of the tumor growth rate was observed. This efficacy is substantially lower than previous reports for closely related proinflammatory immunocytokines (e.g. F8-TNF-α) and argues against translation of IL-1β-F8 into clinical trials at the moment.85
IL-2 fusion constructs
IL-2 is a potent stimulator of the immune system with a pleiotropic set of roles. Its functional repertoire comprises the activation of natural killer (NK) cells, monocytes and cytotoxic T-cells as well as the induction of lymphokine-activated killer cells. Hence, IL-2 can unfold its anti-tumor effect either directly or indirectly by inducing apoptosis of tumor cells or recruiting cytotoxic cells such as NK cells, macrophages, and cytotoxic T lymphocytes (CTL).122 Structurally, IL-2 is essentially a four-helix bundle protein that is stabilized by a disulfide bond and interconnected by short loops. The IL-2 family also comprise five additional cytokines (IL-4, IL-7, IL-9, IL-15, and IL-21) which all share a common receptor subunit (IL-2 receptor γ chain) and play a major role in promoting and maintaining T lymphocyte populations.19 IL-2 has been shown to have a profound effect on metastatic cancer at the expense of severe toxicities such as capillary leak syndrome, hypotension, fever, and nausea.18,123 Therefore, it is the most extensively researched cytokine in terms of different immunocytokine fusion formats with vast preclinical and clinical trial data available. A considerable number of different formats were evaluated and their performance comprehensively reviewed elsewhere.8,25,35,60,124,125 Initial attempts to combine IL-2 with an anti-placental alkaline phosphatase (PLAP) scFv targeting PLAP-expressing tumors have been undertaken more than two decades ago.86,87 Since then, the cytokine has been fused to many single-chain fragments targeting a diverse selection of tumor antigens such as EDA and EDB splice variants of fibronectin, TNC A1, EpCAM, and α-folate receptor (α-FR).88–90,94,99 In these studies, antigen specificity and bioactivity of the fusion constructs were retained upon purification and beneficial anti-tumor effects were demonstrated in mouse tumor models. As an example, the EDA-targeting immunocytokine F8-IL-2 induced substantial local changes within immune effector cell populations in a metastatic murine model of non-small cell lung cancer (NSCLC), demonstrating its potential as monotherapy for the treatment of this indication.90
Notably, attempts have been made to fuse the F8-IL-2 cytokine with the disulfide-linked maytansinoid DM1 microtubular inhibitor in order to create a trifunctional immunocytokine-drug conjugate (IDC). In a syngeneic C1498 mouse model of AML chloromas, the IDC demonstrated potent therapeutic activity at very low doses and even cured some of the treated mice. Thus, it has been suggested that the combination of chemotherapeutics and immunocytokines in a single molecular entity could be of potential value for the therapy of AML in future clinical studies.126
In terms of therapeutic efficacy, the most promising and advanced candidates among scFv-based IL-2 immunocytokines recognize the EDB variant of fibronectin (scFv L19 – Darleukin) and the alternatively spliced A1 domain of TNC (scFv F16 – Teleukin), respectively.99,127 Both constructs were developed in the non-covalent, homodimeric diabody format and are owned by the Italian-based biotech company Philogen (http://www.philogen.com/en/). L19-IL-2 was evaluated as a single agent against metastatic renal cell carcinoma (mRCC) and combined with dacarbazine for the treatment of metastatic melanoma in clinical phase I studies.91,92 When used as a monotherapeutic, 83% of mRCC patients showed stable disease after two cycles of treatment. Combination therapy with dacarbazine induced a 28% objective response among 29 metastatic melanoma patients. Based on these initial results, L19-IL-2 was further tested in phase II clinical trials in patients with metastatic melanoma alone95 and in combination with another immunocytokine (L19-TNF-α; Fibromun).51 Currently, the combinatorial treatment of malignant melanoma with Darleukin and Fibromun is evaluated in clinical phase III trials (NCT02938299). Moreover, L19-IL-2 combination with rituximab (anti-CD20) is currently being assessed in clinical phase I/II trials (NCT02957019) based on encouraging preclinical results using localized and systemic xenograft models of NHL where a complete eradication of the B-cell lymphoma xenografts was observed.93 Apart from being useful in the treatment of certain cancers, the L19-IL-2 fusion protein has also been tested in a mouse model of atherosclerosis where it produced rapid shrinkage of atherosclerotic plaques via expansion of regulatory T-cells.128
F16-IL-2 has been proven valuable in combination with the cytostatic chemotherapeutics doxorubicin or paclitaxel for the treatment of various types of cancer.96,98 Dose escalation studies within phase Ib/II clinical trials have been performed involving patients with advanced solid tumors and metastatic breast cancer, respectively. These studies yielded a recommended dose of 25 MIU F16-IL-2 which can be safely and repeatedly administered.97 Furthermore, efficiency in a hematologic setting has been reported targeting acute myeloid leukemia (AML). Four patients with relapsed AML after allogeneic hematopoietic stem cell transplantation experienced encouraging responses upon infusion of F16-IL-2, owing to an extensive infiltration of immune effector cells in the bone marrow.52 Phase I clinical trials of F16-IL-2 in combination with cytarabine are currently in planning (NCT02957032). Furthermore, combinatorial treatment of AML with F16-IL-2 and the CD33 targeting antibody BI 836858 is currently administered in a phase I clinical trial to find and investigate a safe dose of both biologics (NCT03207191).
IL-4 fusion constructs
IL-4 is a potent regulator of humoral and adaptive immune responses and acts by stimulating proliferation of activated B-cell and T-cells. IL-4 is a member of the IL-2 cytokine family and signals through the IL-4 receptor alpha chain (IL-4Rα) which dimerizes with the common gamma chain on hematopoietic cells or with the IL-13 receptor alpha 1 (IL-13Rα1) on non-hematopoietic cells.129 In general, IL-4 is closely related to IL-13 as the two cytokines share many biological and immunoregulatory functions on B-lymphocytes, monocytes, dendritic cells (DCs), and fibroblasts.130 Accordingly, a significant similarity in their folding topology was observed.131 In view of its anti-inflammatory effect, IL-4 represents an attractive payload for immunocytokines in certain inflammatory and autoimmune disorders. Consequently, IL-4 has been fused to an antibody in the diabody format that targets the alternatively spliced A domain of fibronectin (F8-IL-4 – Tetravil). This construct has been initially found to be therapeutically active in immunocompetent murine models of skin inflammation, confirming previous observations of beneficial effects of non-targeted IL-4 in patients suffering from psoriasis.66 Promising results were also obtained using F8-IL-4 in preclinical studies in mice with experimentally induced endometriosis in which a significant inhibition of the development of endometriotic lesions was observed.103 Moreover, the cytokine has been shown to selectively localize to arthritic sites in a collagen-induced RA model in mice. Most importantly, when used in combination with the glucocorticoid dexamethasone, 100% of treated mice showed a complete remission of the disease. A cytokine analysis of the paws of the animals at the end of the experiment confirmed that the treatment caused a durable normalization of cytokine concentrations, indicating the absence of inflammatory processes.100,102 Fueled by these encouraging results, Tetravil is currently being prepared for clinical testing in patients suffering from endometriosis and RA.132
Besides its usefulness in non-oncological pathologies, F8-IL-4 also inhibited tumor growth in three different immunocompetent mouse cancer models. Furthermore, combination therapy with an IL-12-based immunocytokine resulted in a complete eradication of solid tumors in the same setting.101
IL-6 fusion constructs
IL-6 is a cytokine with a pleiotropic set of immunomodulatory functions and confers context-dependent pro- and anti-inflammatory effects. Thus, IL-6 has been considered as an interesting target for pharmacological applications. It is synthesized by almost all stromal and immunological cells with IL-1β and TNF-α being major inducers of expression.133 Apart from being an important factor in host defense mechanism, it is as well associated with the pathology of many inflammatory disorders and autoimmune diseases such as RA and psoriasis.134 It has been demonstrated that untargeted systemic application of recombinant IL-6 generates a beneficial response in syngeneic murine cancer models with an even more pronounced effect when used in combination with sub-therapeutic doses of TNF-α.135 By contrast, high IL-6 serum levels and tumor promoting activities have been repeatedly reported. Consequently, current preclinical and clinical studies are predominantly designed to explore blocking of IL-6 signaling pathways as potential anti-cancer strategies.136 In fact, only one IL-6-based immunocytokine has so far been characterized in therapeutic studies in an immunogenic murine model of cancer, employing the F8 antibody in diabody format as targeting moiety. However, the F8-IL-6 construct exhibited only a modest inhibitory effect on the tumor growth rate (<50%) and a poor increase in survival.85
IL-7 fusion constructs
IL-7 is considered to be a potent pro-inflammatory immune regulator. It is mainly produced by stromal cells in the bone marrow and thymus rather than in leukocytes. Nevertheless, small amounts are also synthesized in DCs and IL-7 has been found to facilitate the development of lymphocytes and regulate naïve and memory CD4 and CD8 T-cell homeostasis. Moreover, elevated levels of the cytokine are associated with certain forms of solid and hematological malignancies. The mechanism of the influence of the protein upon tumor cell expansion is not yet fully understood.137,138 IL-7 signals through the IL-7 receptor (IL-7R) which is a heterodimeric complex of the IL-7R α-chain and the common receptor γ-chain.139
Initial attempts to fuse the murine IL-7 (mIL-7) to the F8 antibody in a non-covalent diabody format (targeting the alternatively spliced EDA domain of fibronectin) resulted in poor biodistribution to grafted F9 teratocarcinomas in athymic and immunocompetent mice. This effect was concentration dependent and led to the conclusion that the fusion protein might get captured by IL-7R in normal tissue. This inadequate tumor targeting was paralleled with an insufficient therapeutic effect. Further efforts to improve the immunocytokine format eventually culminated in a product with the F8 antibody fragment fused to the N- and C-terminus of mIL-7 resulting in a bispecific fusion construct comprising one mIL-7 molecule (F8-mIL7-F8). However, statistically significant retardation of growth of the F9 carcinomas could only be achieved in immunocompetent mice. These effects were also observed when coupling IL-7 to an scFv in the same format but with irrelevant specificity. Hence, IL-7 might only exhibit limited potential to serve as a payload in immunocytokine applications.104
IL-9 fusion constructs
IL-9 is a small (126 residues without the signal peptide) cytokine that is secreted by T helper cells and acts on a variety of hematocytes in a regulatory fashion. Notably, the human IL-9 sequence comprises four distinct N-glycosylation sites, while O-linked glycans seem to be absent. This marks it as considerably different from other cytokines.105 IL-9 functions through interaction with the IL-9 receptor (IL-9R) in complex with the common cytokine receptor γ-chain subunit.140 IL-9 was shown to mediate a robust tumor immunity response to B16F10 melanoma in mice.141 However, IL-9 levels were also linked to increased proliferation rates in murine T and B cell lymphomas and further associated with myeloid malignancies and Hodgkin disease.142,143 To further evaluate the T-cell mediated anticancer potential of the cytokine, IL-9 was fused to the C-terminus of the F8 diabody. Surprisingly, biodistribution analysis of the resulting immunocytokine suggested that efficient targeting of a subcutaneously grafted F9 teratocarcinoma in mice is highly dependent on the production method of the fusion protein construct. While transient expression methods in CHO cells yielded a product that selectively localized to the tumor, protein expressed from stably transfected cell lines failed to do so. This was explained with a difference in the glycosylation profile of the two otherwise identical constructs and highlights the importance of glycosylation in terms of biodistribution and systemic clearance of immunocytokine products. No data on the efficacy of F8-IL-9 regarding tumor growth inhibition were reported.105
IL-10 fusion constructs
IL-10 is an important anti-inflammatory cytokine and exhibits a strong immunosuppressive activity profile. It is a prominent member of the IL-10 family of cytokines which further includes IL-19, IL-20, IL-22, IL-24, IL-26 and type I, II, and III interferons.144 While IL-10 is present as a homodimer in solution, two IL-10 dimers are needed to activate the corresponding IL-10 receptor which consists of two molecules of the high affinity IL-10 receptor 1 chain and two molecules of the low affinity IL-10 receptor 2 chain.145 IL-10 is a cytokine with a pleiotropic set of functions and has a profound impact on immunoregulation and inflammation, with autoimmunity evolving in its absence. Secreted by diverse T-cell subpopulations, B-cells, macrophages, monocytes, DCs, NK cells, mast cells, neutrophils and eosinophils, IL-10 primarily acts on monocytes where it regulates the production of MHC class II and costimulatory molecules. Furthermore, it downregulates the expression of IL-1β and TNF, thus counterbalancing a pro-inflammatory response.146
There are basically two roles of IL-10 in immune-mediated diseases. On one hand, disorders can be caused by an overproduction of the cytokine which results in undesired immuno-suppressive effects that can promote growth of certain types of cancer. Examples include lupus erythematosus, EBV-associated lymphomas, and skin malignancies such as melanoma.147,148 On the other hand, a relative or absolute IL-10 deficiency translates into a persistent activation of the immune system as seen in chronic inflammatory bowel disease, psoriasis, and RA.149 Consequently, pharmacologic effects of IL-10 were explored in a handful of chronic inflammatory disorders. While treatment of mice with type 2 collagen-induced arthritis yielded some promising results, recombinant human IL-10 did not show any improvement in RA patients.150–152 In an attempt to overcome some of the clinical limitations of the cytokine, IL-10 was fused to an scFv derived from the L19 antibody (recognizes the alternatively spliced extra-domain B of fibronectin). Upon intravenous administration in a murine model of RA, this immunocytokine exhibited superior activity when compared to untargeted IL-10 and led to an improvement of the arthritic score and a reduction of paw swelling.61 By contrast, while L19-IL-10 was shown to specifically target chronically inflamed skin in vivo, no improvement of inflammatory processes could be detected in the same mouse model.62
Another immunocytokine targeting the extra-domain A of fibronectin (F8-IL-10 – Dekavil) showed even more promising results in dose escalating studies, particularly when used in combination with methotrexate (MTX) in RA patients. Signs of beneficial therapeutic effects were already observed at low drug doses in clinical phase Ib trials with no dose limiting toxicities (DLTs) or adverse events recorded.153 At present, F8-IL-10 is further investigated in phase I clinical trials to study its safety and efficacy when administered subcutaneously in combination with MTX (NCT02076659).
Moreover, virally encoded IL-10 (vIL-10) fused to an antibody fragment (1–11E) against ROS-CII was shown to specifically localize to inflamed arthritic joints.68 Treatment efficiency in a mouse model was stable for several days and serum levels of pro-inflammatory cytokines were significantly lower compared to the control group treated with an vIL-10 immunocytokine of irrelevant specificity. Additionally, an accelerated reduction of knee-joint swelling and redox state was observed.107
IL-12 fusion constructs
IL-12 is a heterodimeric pro-inflammatory cytokine and represents an important link between innate resistance and adaptive immunity. It is mainly produced by DCs and phagocytes as a response to invading pathogens during infections where it regulates NK cells and T-cell activation. IL-12, together with IL-2 initiates differentiation of TH1 which in turn induces production of the main effector cytokine IFN-γ. The cytokine signals through the IL-12 receptor which is composed of the two distinct subunits IL-12Rβ1 and IL-12Rβ2. IL-12 is the eponymous cytokine of the IL-12 family which further includes the heterodimeric members IL-23, IL-27, and IL-35 that partly share their individual subunits amongst each other. IL-12 comprises the p40 and p35 subunit which are interconnected and stabilized by a disulfide bond.20,154,155 With regard to its ability to activate both innate and adaptive immunities, IL-12 appeared to represent a promising candidate for cancer immunotherapy. However, only low anti-tumor effects were observed in clinical trials which were often paralleled with unacceptable toxicity levels. Thus, the development of more targeted approaches seemed plausible.156
Due to its heterodimeric nature, development of IL-12-based immunocytokines turned out to be rather challenging as the end products were prone to form high molecular weight aggregates that are biologically inactive. Thus, various immunocytokine formats have been developed in which both subunits are genetically connected by a peptide linker. This single chain IL-12 construct was linked to the scFv of the L19 antibody, specific for the EDB variant of fibronectin. Compared to an IL-12-based immunocytokine of irrelevant specificity, L19-IL-12 displayed markedly superior anti-tumor activity in a murine lung-metastasis model and in two different subcutaneous tumor models in immunocompetent animals. The treatment induced recruitment of macrophages, lymphocyte-activate killer (LAK) cells, NK cells, and T lymphocytes to the tumor environment, and stimulated IFN-γ secretion in the tumor mass and blood.157 When used in combination with the homotrimeric immunocytokine L19-TNF-α, anti-tumor activity of L19-IL-12 could even be potentiated.108 Further improvement could be achieved by constructing a bispecific variant of the L19-IL-12 construct with one scFv fused to both, the p35 and the p40 subunits (L19-p35-p40-L19).29
A similar approach was chosen when combining the EDA variant of fibronectin targeting antibody fragment F8 and IL-12. In this construct, the p35 and p40 subunits were separately fused to one F8 scFv each and supplied on two distinct expression vectors to form a heterodimeric and bispecific immunocytokine via the original disulfide bond in vivo. Despite the biochemical challenges of this strategy in terms of gene dosage effects and correct heterodimer formation, selective accumulation at the vascular structures of the tumor and excellent tumor uptake of F8-IL12-F8 was described.109 In a follow-up study, the initial construct was compared to similar immunocytokines either comprising one IL-12 heterodimer and two distinct F8 scFv moieties (IL12-F8-F8) or two IL-12 heterodimers and F8 in a (noncovalent) diabody format (IL12-F8-F8-IL12), respectively. While the diabody construct failed to localize at the tumor site, a murine variant of IL12-F8-F8 mediated significant tumor retardation in immunocompetent mice bearing subcutaneous F9 teratocarcinoma. Moreover, combination treatment with paclitaxel resulted in long-lasting tumor growth control with 50% of the treated mice being cured.110
An enhanced anti-tumor immune response was also observed when targeting IL-12 to HER2 (also known as ErbB-2) which is frequently overexpressed in epithelial cancer cells. Intramuscular electrogene transfer of an expression vector coding for a antiHER2scFv-IL-12 fusion construct in a syngeneic murine bladder tumor model resulted in significantly reduced tumor growth and prolonged survival of the treated animals. Again, elevated IFN-γ levels and increased infiltration of T lymphocytes were observed.111
IL-12 has also been investigated in combination with a CD30-targeting antibody fragment (HRS3-IL12). Binding to CD30+ H/RS cells was confirmed and the therapeutic suitability of the construct against Hodgkin lymphoma was suggested.112
Another IL-12-based immunocytokine in scFv format (IL12-SS1) was developed against the membrane-anchored glycoprotein mesothelin which is abundantly expressed at the cell surface of malignant mesothelioma. The bioactivity of the resulting product on established mesothelioma was investigated in a nude mouse model where it caused significantly retarded tumor growth. However, despite the findings that the activity of the insect cell-produced fusion protein was not significantly higher than an immunotoxin based on the same scFv fragment, white blood cell levels were elevated by about two-fold upon administration of the immunocytokine.113
Surprisingly, despite the vast amount of preclinical data available for IL-12-based immunocytokines in the scFv format, only two constructs harboring whole IgG antibodies (BC1-IL-12 and NHS-IL-12) have advanced to the clinical trial phase so far (NCT02994953 and NCT01417546 for NHS-IL-12 trials in solid tumors).158,159
IL-13 fusion constructs
IL-13 has a variety of immunoregulatory functions and is mainly produced by activated TH2 lymphocytes. While it was initially thought to be functionally redundant to IL-4, it became clear that IL-13 is a crucial mediator in the pathogenesis of allergic inflammation with a number of distinct functions. The cytokine signals through the common IL-4Rα subunit but additionally requires binding of the proteins IL-13Rα1 and IL-13Rα2 to constitute a fully functional signal transduction complex.160 The IL-13 receptor has been found on multiple cell types such as B-cells, basophils, eosinophils, mast cells, monocytes, macrophages, fibroblasts, endothelial and mucosal epithelial cells and smooth muscle cells but, unlike the IL-4 receptor complex, seems to be absent from T- cells. Consequently, IL-13 seems to be involved in the effector phase of an allergic response rather than in initial differentiation of CD4+ lymphocytes in TH2-type cells.161 Application of IL-13 has been shown to exhibit beneficial effects on tumor growth by inhibition of cell proliferation in several different cancer models.162,163 Thus, the cytokine was considered as a promising payload in the immunocytokine format.
Murine IL-13 (m-IL13) was genetically fused to the F8 antibody in the diabody format, targeting the oncofetal EDA domain of fibronectin. In this construct, the cytokine payload was attached to the C-terminus of the variable light chain, giving rise to mIL13-F8-F8-mIL13 (subsequently referred to as F8-IL13 for simplicity). In accordance with similar F8-based immunocytokine constructs, F8-IL13 was found to selectively localize to tumor blood vessels in two immunocompetent mouse cancer models (F9 teratocarcinoma and Wehi-164 fibrosarcoma). It exhibited a potent anti-tumor activity but failed to cure the cancer completely. When administered in combination with murine IL12-F8-F8, however, a long-lasting eradication of both tumors could be observed in a high proportion of treated animals. Furthermore, rejection of the Wehi-164 fibrosarcomas in the corresponding cohort of mice suggested induction of a protective immune response. In vivo depletion experiments revealed that F8-IL13 mediated the anti-tumor activity primarily through activation of CD4+ T-cells.114
IL-15 fusion constructs
IL-15 features a potent pro-inflammatory activity and is expressed in a considerable number of different tissues. However, translation of the cytokine is mainly limited to monocytes, macrophages, and DCs. IL-15 is a member of the IL-2 family of cytokines and interacts with the heterotrimeric IL-15 receptor. This receptor complex is composed of the IL-15 specific α subunit, the β subunit (shared with IL-2), and the γ subunit (shared with IL-2, IL-4, IL-7, IL-9, and IL-21). As a consequence of sharing common receptor components, IL-2 and IL-15 display certain functional redundancies which include stimulation of activated T-cell proliferation and augmented production of immunoglobulins in B-cells.164 Notably, IL-15 induces proliferation of NK cells and facilitates survival of stem, central, and effector memory CD8+ T-cells. Thus, it was considered as a promising cytokine for the treatment of various metastatic malignancies. Although IL-15 has a better safety profile compared to IL-2 in preclinical and phase I clinical trials, various potential side-effects such as atherogenic effects, induction of systemic inflammation as well as autoimmunity and metastasis of certain tumor cells were identified upon treatment with the cytokine.165 Several immunocytokines with whole IgG and scFv antibodies as targeting moiety have been designed and tested in preclinical trials towards their anti-tumor activity so far.115–117,166–168 One fusion protein employed the L19 derived single-chain fragment to specifically deliver the cytokine payload to the oncofetal EDB variant of fibronectin.115 IL-15 was linked to either the N- or C-terminal part of the antibody fragment present in the homodimeric diabody format (IL15-L19 and L19-IL15, respectively). Despite displaying a very similar biochemical behavior, only the C-terminal fusion (L19-IL15) showed biological activity comparable to recombinant IL-15 in a cytokine-dependent cell proliferation assay. Deglycosylated IL15-L19, however, was active in the same assay suggesting that a different glycosylation profile influenced bioactivity of the N-terminal fusion construct. Biodistribution of the two proteins was somewhat similar with a slightly better tumor uptake and tumor to blood ratio for L19-IL15. Consequently, only L19-IL15 was pursued in further studies. In order to evaluate the therapeutic value, the immunocytokine was tested in two different immunocompetent mouse models bearing the F9 teratocarcinoma and the C51 colon adenocarcinoma. L19-IL15 achieved significant tumor control in both cancer models and exhibited a clear anti-metastatic activity, particularly in F9 tumors. In vivo T-cell depletion experiments revealed that CD8+ but not CD4+ lymphocytes are crucial for the beneficial effects of this immunocytokine construct.115
In an attempt to enhance the stimulatory potential of IL-15, Kermer et al.116 designed immunocytokines comprising a FAP targeting scFv, an extended IL-15Rαsushi domain (RD) and IL-15 (scFv-RD-IL-15). Another construct additionally included the costimulatory molecule 4–1BB of the TNF receptor superfamily yielding a trifunctional fusion protein (RD-IL-15-scFv-4–1BB).117 In vivo analysis of antitumor effects in a B16-FAP lung metastasis mouse model revealed that scFv-RD-IL-15 was superior to similar constructs without the targeting moiety or the RD component.116 In regard of enhanced proliferation of activated T-cells and expansion of cytotoxic T-cells in vitro, scFv-RD-IL-15 and the trifunctional construct yielded comparable results. However, targeted 4–1BB costimulation was beneficial when the stimulatory activity of IL-15 was limited. Furthermore, the number of lung metastases after treatment with the trifunctional fusion protein was significantly reduced compared to what was achieved with scFv-RD-IL-15 alone.117
IL-17 fusion constructs
IL-17A (herein referred to as IL-17) is a homodimeric cytokine with pro-inflammatory activity during extracellular bacterial and fungal infections. Furthermore, excess production has been found in many inflammatory and autoimmune disorders. It is the prototype of the IL-17 cytokine family that further includes IL-17B to IL-17F.169 IL-17 is secreted by numerous lymphocytes including TH17 cells, γδT cells, NK cells, and NKT cells. Its biological actions comprise the recruitment and expansion of monocytes as well as neutrophils. Additionally, it enhances the local inflammatory environment by facilitating the production of IL-6 as well as prostaglandin E2 (PGE2) and stimulates T-cell responses. IL-17 elicits its effects through interaction with the IL-17 receptor that is composed of the A and the C chain. Surprisingly, the IL-17 system including the IL-17 receptor appears to be quite unique as it does not resemble any other cytokine family.170 While IL-17 in particular and the IL-17 pathway in general are recognized as targets in the treatment of chronic inflammatory disorders, its role as a therapeutic agent in cancer-related diseases is still controversial.171,172 With this ambiguity in mind, an IL-17-based immunocytokine harboring the single chain fragment of the F8 antibody (specific to EDA of fibronectin) was constructed to investigate potential anti-tumor effects in a targeted approach. In this study, murine IL-17 (mIL-17) was fused to the C-terminus of the scFv, forming a stable homodimer via disulfide linkage upon expression. The bispecific construct was shown to retain binding affinity of the parental antibody and preferably localized to the tumor mass in immunocompetent mice bearing a subcutaneous F9 teratocarcinoma. Although F8-IL17 stimulated tumor angiogenesis and leukocyte infiltration into the tumor mass, no detectable anti-cancer activity was observed compared to the saline control group. In conclusion, employing IL-17 as payload seems of limited use in anti-cancer applications but might prove valuable in stimulating angiogenesis if therapeutically required.118
IL-22 fusion constructs
IL-22 is a pro-inflammatory cytokine that plays an important role in host defense at mucosal surfaces as well as in tissue repair and wound healing. It is part of the IL-10 cytokine family and signals through the heterodimeric IL-22 receptor (IL-22R) complex that is composed of the IL-22R1 and the IL-10R2 subunits.173 Even though it is produced by immune cells such as T-helper subsets and innate lymphocytes, it predominantly acts on non-hematopoietic stromal and epithelial cells. Examples include mainly cells at outer-body barriers in the skin as well as in the digestive and respiratory tract but also in the liver, kidney, and joints.174 While IL-22 generally acts to strengthen epithelial barrier functions, uncontrolled and disproportionate systemic release has been shown to associate with certain pathologies such as psoriasis-like skin inflammation or increased proliferation of tumor cells.175–177 Most notably, the pancreas has been found to be the tissue with the highest levels of IL-22R expression.178 There, IL-22 triggers a protective and anti-apoptotic response which might be useful during the treatment of pancreatitis.179 Furthermore, Hasnain et al.180 demonstrated that IL-22 is able to effectively suppress oxidative and endoplasmic reticulum stress in pancreatic islets. Systemic administration of the cytokine exhibited multiple beneficial effects in a murine high-fat diet (HFD)-induced type 2 diabetes model and led to a fully restored glucose homeostasis and restoration of insulin sensitivity.180 Similarly, Wang et al.181 showed that an IL-22-Fc immunocytokine with a prolonged half-life restored glucose control in both HFD-induced and leptin-receptor deficient murine models of type 2 diabetes. However, prolonged administration of high IL-22 doses in human patients might potentially lead to deleterious off-target effects in other tissues such as increased and uncontrolled cell proliferation in the gut and skin. Thus, work is underway to design and test immunocytokine fusions to minimize off target-effects, while retaining therapeutic efficacy of IL-22 in type 2 diabetes.
In another instance, IL-22 was reported to be beneficial in a murine model of colitis.182 In order to harness and evaluate the pharmacological potential of IL-22 in a targeted manner, immunocytokines featuring the F8 scFv were constructed and therapeutic activity was assessed in mice with dextran sodium sulfate-induced colitis. While N- and C-terminal fusions exhibited equal biochemical characteristics, the N-terminal construct (F8-IL-22) revealed a more selective targeting performance and conferred a faster and more sustained disease recovery.119 A more frequent dosing regimen could further improve the positive effect of F8-IL22 and pave the way for safety pharmacological studies in non-human primates.119
Concluding remarks
The use of immunocytokines for therapeutic purposes is a fast evolving field that has seen the development of various fusion constructs in different topological arrangements. Apart from selecting an appropriate cytokine payload and antigen target, the choice of the antibody format as the directing moiety is of great importance and can have a profound impact on the performance of the whole construct. In general, there appears to be a balance, amongst reported studies, between the usage of whole mAbs and antibody fragments in the preclinical and clinical trial phase. In fact, five out of nine immunocytokines currently investigated in clinical trials employ IgG as the targeting component. Table 2 provides an overview about immunocytokines currently investigated in active clinical trials. However, scFvs and particularly noncovalent diabody formats are becoming increasingly popular as they combine the advantages of a smaller molecule and improved tissue infiltration, with benefits of augmented avidity. In the great majority of cases, immunocytokines are directed against oncological targets but potential applications in chronic inflammatory diseases and autoimmune disorders are gaining increasing attention. Most notably, an IL-4 containing construct led to the complete remission of RA when administered in combination with dexamethasone in a murine model and is now to be tested in clinical trials.102 Principally, cytokine delivery could be achieved in virtually any pathology where immunomodulatory effects, leukocyte recruitment, and to a smaller extent, tissue homeostasis is considered therapeutically advantageous. Shuttling IL-22 to pancreatic β-cells in order to alleviate oxidative and ER stress symptoms such as poor glycemic control might serve as an example for the latter case.180 Furthermore, combination of immunocytokines with other therapeutic modalities can greatly enhance the pharmacological effects of the individual partners in a synergistic manner. Consequently, immunocytokines were and are administered in combination with conventional chemotherapeutics or anti-inflammatory drugs in a number of past and current clinical trials. Most notably, combinations of different immunocytokines harboring cytokine payloads with complementary functions have yielded some promising results. This approach is propelled by the observation that bifunctional fusion proteins comprising granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukins can exhibit unique signaling and unexpected biological effects.183 These “fusokines” exert their mode of action by coupling activated GM-CSF and interleukin receptors together which subsequently triggers unprecedented downstream signaling events.184 Thus, this specific modulation of the immune response has shown great promise as a potential treatment of certain cancer and autoimmune conditions.185,186
Table 2.
Overview of immunocytokines currently investigated in active clinical trials.
| Name | Format (target) | Clinical trial phase (indication) | NCT# |
|---|---|---|---|
| IL-2 | |||
| Cergutuzumab Amunaleukin | IgG (CEA) | Phase Ib + atezolizumab (solid tumors) | NCT02350673 |
| F16-IL2 (Teleleukin) | Diabody (TNC-A) | Phase I + BI 836858 (AML)Phase I + cytarabine (AML) | NCT03207191NCT02957032 |
| hu14.18-IL2 | IgG (GD2) | Phase II (stage III/IV melanoma)Phase I + NK cells (neuroblastoma)Phase II + chemotherapy (neuroblastoma) | NCT00590824NCT03209869NCT01857934 |
| L19-IL2 (Darleukin) | Diabody (EDB-FN) | Phase I/II + rituximab (DLBCL)Phase III + L19-TNFα (melanoma) | NCT02957019NCT02938299 |
| RO6874281 | IgG (FAP) | Phase I (metastatic solid tumors)Phase Ib + atezolizumab/bevacizumab (mRCC) | NCT02627274NCT03063762 |
| IL-10 | |||
| F8-IL10 (Dekavil) | Diabody (EDA-FN) | Phase II + methotrexate (rheumatoid arthritis) | NCT02270632 |
| IL-12 | |||
| NHS-IL12 (M9241) | IgG (DNA) | Phase I (solid tumors)Phase I + avelumab (solid tumors) | NCT01417546NCT02994953 |
| IFN-α | |||
| IGN002 | IgG (CD20) | Phase I (NHL)Phase II (NHL) | NCT02519270NCT02847949 |
| TNF-α | |||
| L19-TNFα (Fibromun) | Trimeric scFv (EDB-FN) | Phase I + doxorubicin (solid tumors)Phase III + L19-IL2 (melanoma) | NCT02076620NCT02938299 |
CEA: carcinoembryonic antigen; TNC: tenascin; GD2: disialoganglioside GD2; DLBCL: diffuse large B cell lymphoma; FN: fibronectin; FAP: fibroblast activation protein; mRCC: metastatic renal cell carcinoma; NHL: non-Hodgkin lymphoma; IFN: interferon; TNF: tumor necrosis factor.
In summary, although great progress has been made in engineering and testing a variety of constructs that employ cytokines in general and interleukins in particular, extensive research is required to identify the most suitable oncological and non-oncological targets for a particular payload and a particular disease. This will be imperative to enable translation of the respective immunocytokine from preclinical into clinical practice.
Authors’ contributions
CF and RTB wrote the manuscript, all authors participated in the review and editing of the manuscript. SK designed all figures.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was funded by NHMRC Development Grant APP1113905 and the University of Queensland. MAM was supported by a NHMRC Principal Research Fellowship. The Translational Research Institute is supported by a grant from the Australian Government.
References
- 1.Ehrlich P, Morgenroth J. Die Seitenkettentheorie der Immunität. Anleitung zu hygenischen Untersuchungen: nach den im Hygenischen Institut der königl. Ludwig-Maximilians-Universität zu München üblichen Methoden zusammengestellt 3 Aufl 1902; 381–394
- 2.Ehrlich P. Aus theorie und praxis der chemotherapie. Folia Serol 1911;697–714 [Google Scholar]
- 3.Li Q, Yi L, Marek P. Antibodies and their multivalent constructs for cancer therapy. Protein Pept Lett 2014; 21:1017–24 [DOI] [PubMed] [Google Scholar]
- 4.Wootla B, Denic A, Rodriguez M. Polyclonal and monoclonal antibodies in clinic. Methods Mol Biol 2014; 1060:79–110 [DOI] [PubMed] [Google Scholar]
- 5.Jerjian TV, Glode AE, Thompson LA, O'bryant CL. Antibody-drug conjugates: a clinical pharmacy perspective on an emerging cancer therapy. Pharmacotherapy 2016; 36:99–116 [DOI] [PubMed] [Google Scholar]
- 6.Wang RE, Liu T, Wang Y, Cao Y, Du J, Luo X, Deshmukh V, Kim CH, Lawson BR, Tremblay MS, Young TS, Kazane SA, Wang F, Schultz PG. An immunosuppressive antibody–drug conjugate. J Am Chem Soc 2015; 137:3229–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graversen JH, Svendsen P, Dagnæs-Hansen F, Dal J, Anton G, Etzerodt A, Petersen MD, Christensen PA, Møller HJ, Moestrup SK. Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Mol Ther 2012; 20:1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lode HN, Xiang R, Becker JC, Gillies SD, Reisfeld RA. Immunocytokines: a promising approach to cancer immunotherapy. Pharmacol Ther 1998; 80:277–92 [DOI] [PubMed] [Google Scholar]
- 9.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci UsaU S A 1990; 87:6934–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters M, Lobie P, Garcia-Aragon J, Rowlinson S, Gobius K, Bastiras S, Robbins A, Zhang C, Young W, Muscat G, Barnard RT. Structure, location and role of the GH receptor. In: Ho K, Werther G, Cowell C (eds) Growth and sexual development. New York: Harwood Academic Press, 1993, pp.3–28.
- 11.Floros T, Tarhini AA. Anticancer cytokines: biology and clinical effects of interferon-alpha2, interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin Oncol 2015; 42:539–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad JJ. Cytokines and related receptor-mediated signaling pathways. Biochem Biophys Res Commun 2002; 297:700–13 [DOI] [PubMed] [Google Scholar]
- 13.Tarhini AA, Gogas H, Kirkwood JM. IFN-α in the treatment of melanoma. J Immunol 2012; 189:3789–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999; 17:2105–16 [DOI] [PubMed] [Google Scholar]
- 15.Friedman RM. Clinical uses of interferons. Br J Clin Pharmacol 2008; 65:158–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9:361–71 [DOI] [PubMed] [Google Scholar]
- 17.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol 2009; 27:83–117 [DOI] [PubMed] [Google Scholar]
- 18.Baldo BA. Side effects of cytokines approved for therapy. Drug Saf 2014; 37:921–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benczik M, Gaffen SL. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol Invest 2004; 33:109–42 [DOI] [PubMed] [Google Scholar]
- 20.Jones LL, Chaturvedi V, Uyttenhove C, Van Snick J, Vignali DAA. Distinct subunit pairing criteria within the heterodimeric IL-12 cytokine family. Mol Immunol 2012; 51:234–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith RA, Baglioni C. The active form of tumor necrosis factor is a trimer. J Biol Chem 1987; 262:6951–4 [PubMed] [Google Scholar]
- 22.Krippner-Heidenreich A, Grunwald I, Zimmermann G, Kuhnle M, Gerspach J, Sterns T, Shnyder SD, Gill JH, Mannel DN, Pfizenmaier K, Scheurich P. Single-chain TNF, a TNF derivative with enhanced stability and antitumoral activity. J Immunol 2008; 180:8176–83 [DOI] [PubMed] [Google Scholar]
- 23.Strohl WR. Fusion proteins for half-life extension of biologics as a strategy to make biobetters. BioDrugs 2015; 29:215–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillies SD, Lan Y, Lo K-M, Super M, Wesolowski J. Improving the efficacy of antibody-interleukin 2 fusion proteins by reducing their interaction with Fc receptors. Cancer Res 1999; 59:2159–66 [PubMed] [Google Scholar]
- 25.Sondel PM, Gillies SD. Current and potential uses of immunocytokines as cancer immunotherapy. Antibodies 2012; 1:149–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hank JA, Surfus JE, Gan J, Jaeger P, Gillies SD, Reisfeld RA, Sondel PM. Activation of human effector cells by a tumor reactive recombinant anti-ganglioside GD2 interleukin-2 fusion protein (ch14.18-IL2). Clin Cancer Res 1996; 2:1951–9 [PubMed] [Google Scholar]
- 27.Niesner U, Halin C, Lozzi L, Gunthert M, Neri P, Wunderli-Allenspach H, Zardi L, Neri D. Quantitation of the tumor-targeting properties of antibody fragments conjugated to cell-permeating HIV-1 TAT peptides. Bioconjug Chem 2002; 13:729–36 [DOI] [PubMed] [Google Scholar]
- 28.Kuo TT, Aveson VG. Neonatal Fc receptor and IgG-based therapeutics. MAbs 2011; 3:422–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gafner V, Trachsel E, Neri D. An engineered antibody-interleukin-12 fusion protein with enhanced tumor vascular targeting properties. Int J Cancer 2006; 119:2205–12 [DOI] [PubMed] [Google Scholar]
- 30.Melkko S, Halin C, Borsi L, Zardi L, Neri D. An antibody-calmodulin fusion protein reveals a functional dependence between macromolecular isoelectric point and tumor targeting performance. Int J Radiat Oncol Biol Phys 2002; 54:1485–90 [DOI] [PubMed] [Google Scholar]
- 31.Thurber GM, Schmidt MM, Wittrup KD. Factors determining antibody distribution in tumors. Trends Pharmacol Sci 2008; 29:57–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farajnia S, Ahmadzadeh V, Tanomand A, Veisi K, Khosroshahi SA, Rahbarnia L. Development trends for generation of single-chain antibody fragments. Immunopharmacol Immunotoxicol 2014; 36:297–308 [DOI] [PubMed] [Google Scholar]
- 33.Colcher D, Pavlinkova G, Beresford G, Booth BJ, Choudhury A, Batra SK. Pharmacokinetics and biodistribution of genetically-engineered antibodies. Q J Nucl Med 1998; 42:225–41 [PubMed] [Google Scholar]
- 34.Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res 1992; 52:3402–8 [PubMed] [Google Scholar]
- 35.Kontermann RE. Antibody–cytokine fusion proteins. Arch Biochem Biophys 2012; 526:194–205 [DOI] [PubMed] [Google Scholar]
- 36.Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM. High affinity restricts the localization and tumor penetration of single-chain Fv antibody molecules. Cancer Res 2001; 61:4750–5 [PubMed] [Google Scholar]
- 37.Kiefer JD, Neri D. Immunocytokines and bispecific antibodies: two complementary strategies for the selective activation of immune cells at the tumor site. Immunol Rev 2016; 270:178–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74 [DOI] [PubMed] [Google Scholar]
- 39.Fang J, Xiao L, Joo K-I, Liu Y, Zhang C, Liu S, Conti PS, Li Z, Wang P. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. Int J Cancer 2016; 138:1013–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonald PC, Winum J-Y, Supuran CT, Dedhar S. Recent developments in argeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 2012; 3:84–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connor JP, Cristea MC, Lewis NL, Lewis LD, Komarnitsky PB, Mattiacci MR, Felder M, Stewart S, Harter J, Henslee-Downey J, Kramer D, Neugebauer R, Stupp R. A phase 1b study of humanized KS-interleukin-2 (huKS-IL2) immunocytokine with cyclophosphamide in patients with EpCAM-positive advanced solid tumors. BMC Cancer 2013; 13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillies SD, Lan Y, Williams S, Carr F, Forman S, Raubitschek A, Lo KM. An anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse model of established human B lymphoma. Blood 2005; 105:3972–8 [DOI] [PubMed] [Google Scholar]
- 43.Albertini MR, Hank JA, Gadbaw B, Kostlevy J, Haldeman J, Schalch H, Gan J, Kim K, Eickhoff J, Gillies SD, Sondel PM. Phase II trial of hu14.18-IL2 for patients with metastatic melanoma. Cancer Immunol Immunother 2012; 61:2261–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rybak JN, Roesli C, Kaspar M, Villa A, Neri D. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res 2007; 67:10948–57 [DOI] [PubMed] [Google Scholar]
- 45.Borgia B, Roesli C, Fugmann T, Schliemann C, Cesca M, Neri D, Giavazzi R. A proteomic approach for the identification of vascular markers of liver metastasis. Cancer Res 2010; 70:309–18 [DOI] [PubMed] [Google Scholar]
- 46.Kumra H, Reinhardt DP. Fibronectin-targeted drug delivery in cancer. Adv Drug Deliv Rev 2016; 97:101–10 [DOI] [PubMed] [Google Scholar]
- 47.Yoshida T, Akatsuka T, Imanaka-Yoshida K. Tenascin-C and integrins in cancer. Cell Adh Migr 2015; 9:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proskuryakov SY, Gabai VL. Mechanisms of tumor cell necrosis. Curr Pharm Des 2010; 16:56–68 [DOI] [PubMed] [Google Scholar]
- 49.van den Heuvel MM, Verheij M, Boshuizen R, Belderbos J, Dingemans AM, De Ruysscher D, Laurent J, Tighe R, Haanen J, Quaratino S. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med 2015; 13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein C, Waldhauer I, Nicolini VG, Freimoser-Grundschober A, Nayak T, Vugts DJ, Dunn C, Bolijn M, Benz J, Stihle M, Lang S, Roemmele M, Hofer T, van Puijenbroek E, Wittig D, Moser S, Ast O, Brünker P, Gorr IH, Neumann S, de Vera Mudry MC, Hinton H, Crameri F, Saro J, Evers S, Gerdes C, Bacac M, van Dongen G, Moessner E, Umaña P. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 2017; 6:e1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danielli R, Patuzzo R, Di Giacomo AM, Gallino G, Maurichi A, Di Florio A, Cutaia O, Lazzeri A, Fazio C, Miracco C, Giovannoni L, Elia G, Neri D, Maio M, Santinami M. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother 2015; 64:999–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schliemann C, Gutbrodt KL, Kerkhoff A, Pohlen M, Wiebe S, Silling G, Angenendt L, Kessler T, Mesters RM, Giovannoni L, Schafers M, Altvater B, Rossig C, Grunewald I, Wardelmann E, Kohler G, Neri D, Stelljes M, Berdel WE. Targeting interleukin-2 to the bone marrow stroma for therapy of acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Cancer Immunol Res 2015; 3:547–56 [DOI] [PubMed] [Google Scholar]
- 53.Lo KM, Lan Y, Lauder S, Zhang J, Brunkhorst B, Qin G, Verma R, Courtenay-Luck N, Gillies SD. huBC1-IL12, an immunocytokine which targets EDB-containing oncofetal fibronectin in tumors and tumor vasculature, shows potent anti-tumor activity in human tumor models. Cancer Immunol Immunother 2007; 56:447–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, Reynolds CP, Kimball J, Albertini MR, Wagner B, Gan J, Eickhoff J, DeSantes KB, Cohn SL, Hecht T, Gadbaw B, Reisfeld RA, Maris JM, Sondel PM. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. JCO 2010; 28:4969–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachanova V, Lansigan F, Quick DP, Vlock D, Gillies S, Nakamura R. Remission induction in a phase I/II study of an anti-CD20-interleukin-2 immunocytokine DI-Leu16-IL2 in patients with relapsed B-Cell lymphoma. Blood 2015; 126:1533 [Google Scholar]
- 56.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thepen T, Huhn M, Melmer G, Tur MK, Barth S. Fcgamma receptor 1 (CD64), a target beyond cancer. Curr Pharm Des 2009; 15:2712–8 [DOI] [PubMed] [Google Scholar]
- 58.Hristodorov D, Mladenov R, Huhn M, Barth S, Thepen T. Macrophage-targeted therapy: CD64-based immunotoxins for treatment of chronic inflammatory diseases. Toxins 2012; 4:676–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw T, Quan J, Totoritis M. B cell therapy for rheumatoid arthritis: the rituximab (anti-CD20) experience. Ann Rheum Dis 2003; 62:ii55–i9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today 2012; 17:583–90 [DOI] [PubMed] [Google Scholar]
- 61.Trachsel E, Bootz F, Silacci M, Kaspar M, Kosmehl H, Neri D. Antibody-mediated delivery of IL-10 inhibits the progression of established collagen-induced arthritis. Arthritis Res Ther 2007; 9:R9–R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trachsel E, Kaspar M, Bootz F, Detmar M, Neri D. A human mAb specific to oncofetal fibronectin selectively targets chronic skin inflammation in vivo. J Invest Dermatol 2007; 127:881–6 [DOI] [PubMed] [Google Scholar]
- 63.Schwager K, Kaspar M, Bootz F, Marcolongo R, Paresce E, Neri D, Trachsel E. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther 2009; 11:R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwager K, Bootz F, Imesch P, Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum Reprod 2011; 26:2344–52 [DOI] [PubMed] [Google Scholar]
- 65.Pedretti M, Rancic Z, Soltermann A, Herzog BA, Schliemann C, Lachat M, Neri D, Kaufmann PA. Comparative immunohistochemical staining of atherosclerotic plaques using F16, F8 and L19: three clinical-grade fully human antibodies. Atherosclerosis 2010; 208:382–9 [DOI] [PubMed] [Google Scholar]
- 66.Hemmerle T, Zgraggen S, Matasci M, Halin C, Detmar M, Neri D. Antibody-mediated delivery of interleukin 4 to the neo-vasculature reduces chronic skin inflammation. J Dermatol Sci 2014; 76:96–103 [DOI] [PubMed] [Google Scholar]
- 67.Bootz F, Schmid AS, Neri D. Alternatively spliced EDA domain of fibronectin is a target for pharmacodelivery applications in inflammatory bowel disease. Inflamm Bowel Dis 2015; 21:1908–17 [DOI] [PubMed] [Google Scholar]
- 68.Hughes C, Faurholm B, Dell'accio F, Manzo A, Seed M, Eltawil N, Marrelli A, Gould D, Subang C, Al-Kashi A, De Bari C, Winyard P, Chernajovsky Y, Nissim A. Human single-chain variable fragment that specifically targets arthritic cartilage. Arthritis Rheum 2010; 62:1007–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. Aaps J 2012; 14:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song S, Yang L, Trepicchio WL, Wyant T. Understanding the supersensitive anti-drug antibody assay: unexpected high anti-drug antibody incidence and its clinical relevance. J Immunol Res 2016;2016:3072586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods 2005; 36:3–10 [DOI] [PubMed] [Google Scholar]
- 72.Xue L, Hickling T, Song R, Nowak J, Rup B. Contribution of enhanced engagement of antigen presentation machinery to the clinical immunogenicity of a human interleukin (IL)-21 receptor-blocking therapeutic antibody. Clin Exp Immunol 2016; 183:102–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopez Hänninen E, Knüver-Hopf J, Atzpodien J. Immunogenicity of recombinant human interleukin-2: biological features and clinical relevance. Biotherapy 1993; 6:251–61 [DOI] [PubMed] [Google Scholar]
- 74.Bertolotto A, Malucchi S, Sala A, Orefice G, Carrieri PB, Capobianco M, Milano E, Melis F, Giordana MT. Differential effects of three interferon betas on neutralising antibodies in patients with multiple sclerosis: a follow up study in an independent laboratory. J Neurol Neurosurg Psychiatry 2002; 73:148–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryff JC. Clinical investigation of the immunogenicity of interferon-alpha 2a. J Interferon Cytokine Res 1997;July(17 Suppl 1):S29–33 [PubMed] [Google Scholar]
- 76.Wadhwa M, Meager A, Dilger P, Bird C, Dolman C, Das RG, Thorpe R. Neutralizing antibodies to granulocyte-macrophage colony-stimulating factor, interleukin-1alpha and interferon-alpha but not other cytokines in human immunoglobulin preparations. Immunology 2000; 99:113–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 2005; 106:1565–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 2004; 304:104–7 [DOI] [PubMed] [Google Scholar]
- 79.Bontrop RE, Otting N, de Groot NG, Doxiadis GG. Major histocompatibility complex class II polymorphisms in primates. Immunol Rev 1999; 167:339–50 [DOI] [PubMed] [Google Scholar]
- 80.Perry LC, Jones TD, Baker MP. New approaches to prediction of immune responses to therapeutic proteins during preclinical development. Drugs R D 2008; 9:385–96 [DOI] [PubMed] [Google Scholar]
- 81.Bryson CJ, Jones TD, Baker MP. Prediction of immunogenicity of therapeutic proteins: validity of computational tools. BioDrugs 2010; 24:1–8 [DOI] [PubMed] [Google Scholar]
- 82.Stickler M, Chin R, Faravashi N, Gebel W, Razo OJ, Rochanayon N, Power S, Valdes AM, Holmes S, Harding FA. Human population-based identification of CD4(+) T-cell peptide epitope determinants. J Immunol Methods 2003; 281:95–108 [DOI] [PubMed] [Google Scholar]
- 83.Mazor R, Kaplan G, Park D, Jang Y, Lee F, Kreitman R, Pastan I. Rational design of low immunogenic anti CD25 recombinant immunotoxin for T cell malignancies by elimination of T cell epitopes in PE38. Cell Immunol 2017; 313:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs 2010; 2:256–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hess C, Neri D. Tumor-targeting properties of novel immunocytokines based on murine IL1beta and IL6. Protein Eng Des Sel 2014; 27:207–13 [DOI] [PubMed] [Google Scholar]
- 86.Boleti E, Deonarain MP, Spooner RA, Smith AJ, Epenetos AA, George AJT. Construction, expression and characterisation of a single chain anti-tumour antibody (scFv)-EL-2 fusion protein. Ann Oncol 1995; 6:945–7 [DOI] [PubMed] [Google Scholar]
- 87.Xiang J, Liu E, Moyana T, Qi Y. Single-chain antibody variable region-targeted interleukin-2 stimulates T cell killing of human colorectal carcinoma cells. Immunol Cell Biol 1994; 72:275–85 [DOI] [PubMed] [Google Scholar]
- 88.Matsumoto H, Liao S, Arakawa F, Ueno A, Abe H, Awasthi A, Kuroki M, Kuroki M. Targeting of interleukin-2 to human MK-1-expressing carcinoma by fusion with a single-chain Fv of anti-MK-1 antibody. Anticancer Res 2002; 22:2001–7 [PubMed] [Google Scholar]
- 89.Melani C, Figini M, Nicosia D, Luison E, Ramakrishna V, Casorati G, Parmiani G, Eshhar Z, Canevari S, Colombo MP. Targeting of interleukin 2 to human ovarian carcinoma by fusion with a single-chain Fv of antifolate receptor antibody. Cancer Res 1998; 58:4146–54 [PubMed] [Google Scholar]
- 90.Wieckowski S, Hemmerle T, Prince SS, Schlienger BD, Hillinger S, Neri D, Zippelius A. Therapeutic efficacy of the F8-IL2 immunocytokine in a metastatic mouse model of lung adenocarcinoma. Lung Cancer 2015; 88:9–15 [DOI] [PubMed] [Google Scholar]
- 91.Eigentler TK, Weide B, de Braud F, Spitaleri G, Romanini A, Pflugfelder A, Gonzalez-Iglesias R, Tasciotti A, Giovannoni L, Schwager K, Lovato V, Kaspar M, Trachsel E, Menssen HD, Neri D, Garbe C. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res 2011; 17:7732–42 [DOI] [PubMed] [Google Scholar]
- 92.Johannsen M, Spitaleri G, Curigliano G, Roigas J, Weikert S, Kempkensteffen C, Roemer A, Kloeters C, Rogalla P, Pecher G, Miller K, Berndt A, Kosmehl H, Trachsel E, Kaspar M, Lovato V, Gonzalez-Iglesias R, Giovannoni L, Menssen HD, Neri D, de Braud F. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer 2010; 46:2926–35 [DOI] [PubMed] [Google Scholar]
- 93.Schliemann C, Palumbo A, Zuberbuhler K, Villa A, Kaspar M, Trachsel E, Klapper W, Menssen HD, Neri D. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood 2009; 113:2275–83 [DOI] [PubMed] [Google Scholar]
- 94.Wagner K, Schulz P, Scholz A, Wiedenmann B, Menrad A. The targeted immunocytokine L19-IL2 efficiently inhibits the growth of orthotopic pancreatic cancer. Clin Cancer Res 2008; 14:4951–60 [DOI] [PubMed] [Google Scholar]
- 95.Weide B, Eigentler TK, Pflugfelder A, Zelba H, Martens A, Pawelec G, Giovannoni L, Ruffini PA, Elia G, Neri D, Gutzmer R, Becker JC, Garbe C. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res 2014; 2:668–78 [DOI] [PubMed] [Google Scholar]
- 96.Braud FGD, Catania C, Masini C, Maur M, Cascinu S, Berardi R, Giovannoni L, Spitaleri G, Boselli S, Neri D. Combinations of the immunocytokine F16-IL2 with doxorubicin or with paclitaxel investigated in phase Ib studies in patients with advanced solid tumors. J Clin Oncol 2010; 28:e13017-e [Google Scholar]
- 97.Catania C, Maur M, Berardi R, Rocca A, Giacomo AM, Spitaleri G, Masini C, Pierantoni C, Gonzalez-Iglesias R, Zigon G, Tasciotti A, Giovannoni L, Lovato V, Elia G, Menssen HD, Neri D, Cascinu S, Conte PF, Braud F. The tumor-targeting immunocytokine F16-IL2 in combination with doxorubicin: dose escalation in patients with advanced solid tumors and expansion into patients with metastatic breast cancer. Cell Adh Migr 2015; 9:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Braud FG, Catania C, Onofri A, Pierantoni C, Cascinu S, Maur M, Masini C, Conte PF, Giovannoni L, Tasciotti A, Lovato V, Neri D, Menssen HD. Combination of the immunocytokine F16-IL2 with doxorubicin or paclitaxel in patients with solid tumors: results from two phase Ib trials. JCO 2011; 29:2595 [Google Scholar]
- 99.Marlind J, Kaspar M, Trachsel E, Sommavilla R, Hindle S, Bacci C, Giovannoni L, Neri D. Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin Cancer Res 2008; 14:6515–24 [DOI] [PubMed] [Google Scholar]
- 100.Hemmerle T, Doll F, Neri D. Antibody-based delivery of IL4 to the neovasculature cures mice with arthritis. Proc Natl Acad Sci U S A 2014; 111:12008–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer 2014; 134:467–77 [DOI] [PubMed] [Google Scholar]
- 102.Kawalkowska JZ, Hemmerle T, Pretto F, Matasci M, Neri D, Williams RO. Targeted IL-4 therapy synergizes with dexamethasone to induce a state of tolerance by promoting Treg cells and macrophages in mice with arthritis. Eur J Immunol 2016; 46:1246–57 [DOI] [PubMed] [Google Scholar]
- 103.Quattrone F, Sanchez AM, Pannese M, Hemmerle T, Vigano P, Candiani M, Petraglia F, Neri D, Panina-Bordignon P. The targeted delivery of interleukin 4 inhibits development of endometriotic lesions in a mouse model. Reprod Sci 2015; 22:1143–52 [DOI] [PubMed] [Google Scholar]
- 104.Pasche N, Woytschak J, Wulhfard S, Villa A, Frey K, Neri D. Cloning and characterization of novel tumor-targeting immunocytokines based on murine IL7. J Biotechnol 2011; 154:84–92 [DOI] [PubMed] [Google Scholar]
- 105.Venetz D, Hess C, Lin C-W, Aebi M, Neri D. Glycosylation profiles determine extravasation and disease-targeting properties of armed antibodies. Proc Natl Acad Sci U S A 2015; 112:2000–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galeazzi M, Baldi C, Schwager K, Neri D, Giovannoni L, Selvi E. FRI0194 A phase IB clinical trial with dekavil (F8-IL10), an anti-inflammatory immunocytokine for the treatment of rheumatoid arthritis, used in combination with methotrexate. Ann Rheum Dis 2013; 71:378 [Google Scholar]
- 107.Hughes C, Sette A, Seed M, D'acquisto F, Manzo A, Vincent TL, Lim NH, Nissim A. Targeting of viral interleukin-10 with an antibody fragment specific to damaged arthritic cartilage improves its therapeutic potency. Arthritis Res Ther 2014; 16:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Halin C, Gafner V, Villani ME, Borsi L, Berndt A, Kosmehl H, Zardi L, Neri D. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res 2003; 63:3202–10 [PubMed] [Google Scholar]
- 109.Sommavilla R, Pasche N, Trachsel E, Giovannoni L, Roesli C, Villa A, Neri D, Kaspar M. Expression, engineering and characterization of the tumor-targeting heterodimeric immunocytokine F8-IL12. Protein Eng Des Sel 2010; 23:653–61 [DOI] [PubMed] [Google Scholar]
- 110.Pasche N, Wulhfard S, Pretto F, Carugati E, Neri D. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin Cancer Res 2012; 18:4092–103 [DOI] [PubMed] [Google Scholar]
- 111.Tsai YS, Shiau AL, Chen YF, Tsai HT, Lee HL, Tzai TS, Wu CL. Enhancement of antitumor immune response by targeted interleukin-12 electrogene transfer through antiHER2 single-chain antibody in a murine bladder tumor model. Vaccine 2009; 27:5383–92 [DOI] [PubMed] [Google Scholar]
- 112.Heuser C, Diehl V, Abken H, Hombach A. Anti-CD30-IL-12 antibody-cytokine fusion protein that induces IFN-gamma secretion of T cells and NK cell-mediated lysis of Hodgkin's lymphoma-derived tumor cells. Int J Cancer 2003; 106:545–52 [DOI] [PubMed] [Google Scholar]
- 113.Kim H, Gao W, Ho M. Novel immunocytokine IL12-SS1 (Fv) inhibits mesothelioma tumor growth in nude mice. PLoS One 2013; 8:e81919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hess C, Neri D. The antibody-mediated targeted delivery of interleukin-13 to syngeneic murine tumors mediates a potent anticancer activity. Cancer Immunol Immunother 2015; 64:635–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-15 and GM-CSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res 2007; 67:4940–8 [DOI] [PubMed] [Google Scholar]
- 116.Kermer V, Baum V, Hornig N, Kontermann RE, Müller D. An antibody fusion protein for cancer immunotherapy mimicking IL-15 trans-presentation at the tumor site. Mol Cancer Ther 2012; 11:1279–88 [DOI] [PubMed] [Google Scholar]
- 117.Kermer V, Hornig N, Harder M, Bondarieva A, Kontermann RE, Müller D. Combining antibody-directed presentation of IL-15 and 4-1BBL in a trifunctional fusion protein for cancer immunotherapy. Mol Cancer Ther 2014; 13:112–21 [DOI] [PubMed] [Google Scholar]
- 118.Pasche N, Frey K, Neri D. The targeted delivery of IL17 to the mouse tumor neo-vasculature enhances angiogenesis but does not reduce tumor growth rate. Angiogenesis 2012; 15:165–9 [DOI] [PubMed] [Google Scholar]
- 119.Bootz F, Ziffels B, Neri D. Antibody-based targeted delivery of interleukin-22 promotes rapid clinical recovery in mice with DSS-induced colitis. Inflamm Bowel Dis 2016; 22:2098–105 [DOI] [PubMed] [Google Scholar]
- 120.Kuno K, Matsushima K. The IL-1 receptor signaling pathway. J Leukoc Biol 1994; 56:542–7 [DOI] [PubMed] [Google Scholar]
- 121.Rinehart J, Hersh E, Issell B, Triozzi P, Buhles W, Neidhart J. Phase 1 trial of recombinant human interleukin-1 beta (rhIL-1 beta), carboplatin, and etoposide in patients with solid cancers: southwest oncology, group study 8940. Cancer Invest 1997; 15:403–10 [DOI] [PubMed] [Google Scholar]
- 122.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 2010; 33:153–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg 1998; 228:307–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Müller D. Antibody fusions with immunomodulatory proteins for cancer therapy. Pharmacol Ther 2015; 154:57–66 [DOI] [PubMed] [Google Scholar]
- 125.List T, Neri D. Immunocytokines: a review of molecules in clinical development for cancer therapy. Clin Pharmacol 2013; 5:29–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.List T, Casi G, Neri D. A chemically defined trifunctional antibody-cytokine-drug conjugate with potent antitumor activity. Mol Cancer Ther 2014; 13:2641–52 [DOI] [PubMed] [Google Scholar]
- 127.Borsi L, Balza E, Bestagno M, Castellani P, Carnemolla B, Biro A, Leprini A, Sepulveda J, Burrone O, Neri D, Zardi L. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer 2002; 102:75–85 [DOI] [PubMed] [Google Scholar]
- 128.Dietrich T, Hucko T, Schneemann C, Neumann M, Menrad A, Willuda J, Atrott K, Stibenz D, Fleck E, Graf K, Menssen HD. Local delivery of IL-2 reduces atherosclerosis via expansion of regulatory T cells. Atherosclerosis 2012; 220:329–36 [DOI] [PubMed] [Google Scholar]
- 129.Gadani SP, Cronk JC, Norris GT, Kipnis J. Interleukin 4: a cytokine to remember. J Immunol 2012; 189:4213–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol 1998; 17:1–52 [DOI] [PubMed] [Google Scholar]
- 131.Moy FJ, Diblasio E, Wilhelm J, Powers R. Solution structure of human IL-13 and implication for receptor binding. J Mol Biol 2001; 310:219–30 [DOI] [PubMed] [Google Scholar]
- 132.Bootz F, Neri D. Immunocytokines: a novel class of products for the treatment of chronic inflammation and autoimmune conditions. Drug Discov Today 2016; 21:180–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16:448–57 [DOI] [PubMed] [Google Scholar]
- 134.Hirano T. Interleukin 6 in autoimmune and inflammatory diseases: a personal memoir. Proc Jpn Acad Ser B Phys Biol Sci 2010; 86:717–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mule JJ, McIntosh JK, Jablons DM, Rosenberg SA. Antitumor activity of recombinant interleukin 6 in mice. J Exp Med 1990; 171:629–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 2014; 141:125–39 [DOI] [PubMed] [Google Scholar]
- 137.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 2011; 11:330–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zarogoulidis P, Lampaki S, Yarmus L, Kioumis I, Pitsiou G, Katsikogiannis N, Hohenforst-Schmidt W, Li Q, Huang H, Sakkas A, Organtzis J, Sakkas L, Mpoukovinas I, Tsakiridis K, Lazaridis G, Syrigos K, Zarogoulidis K. Interleukin-7 and Interleukin-15 for cancer. J Cancer 2014; 5:765–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol 2012; 24:209–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmitt E, Bopp T. Amazing IL-9: revealing a new function for an “old” cytokine. J Clin Invest 2012; 122:3857–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, Clark RA, Kupper TS. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med 2012; 18:1248–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Knoops L, Renauld JC. IL-9 and its receptor: from signal transduction to tumorigenesis. Growth Factors 2004; 22:207–15 [DOI] [PubMed] [Google Scholar]
- 143.Renauld JC, van der Lugt N, Vink A, van Roon M, Godfraind C, Warnier G, Merz H, Feller A, Berns A, Van Snick J. Thymic lymphomas in interleukin 9 transgenic mice. Oncogene 1994; 9:1327–32 [PubMed] [Google Scholar]
- 144.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 2004; 22:929–79 [DOI] [PubMed] [Google Scholar]
- 145.Moore KW, de Waal Malefyt R, Coffman RL, O'garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19:683–765 [DOI] [PubMed] [Google Scholar]
- 146.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev 2010; 21:315–24 [DOI] [PubMed] [Google Scholar]
- 147.Yue FY, Dummer R, Geertsen R, Hofbauer G, Laine E, Manolio S, Burg G. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int J Cancer 1997; 71:630–7 [DOI] [PubMed] [Google Scholar]
- 148.Grondal G, Kristjansdottir H, Gunnlaugsdottir B, Arnason A, Lundberg I, Klareskog L, Steinsson K. Increased number of interleukin-10-producing cells in systemic lupus erythematosus patients and their first-degree relatives and spouses in Icelandic multicase families. Arthritis Rheum 1999; 42:1649–54 [DOI] [PubMed] [Google Scholar]
- 149.Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev 2010; 21:331–44 [DOI] [PubMed] [Google Scholar]
- 150.Hurst NP. Combination therapy in rheumatoid arthritis. Rheumatology 1999; 38:789–90 [DOI] [PubMed] [Google Scholar]
- 151.Walmsley M, Katsikis PD, Abney E, Parry S, Williams RO, Maini RN, Feldmann M. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis Rheum 1996; 39:495–503 [DOI] [PubMed] [Google Scholar]
- 152.Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J Clin Invest 1995; 95:2868–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galeazzi M, Bazzichi L, Sebastiani GD, Neri D, Garcia E, Ravenni N, Giovannoni L, Wilton J, Bardelli M, Baldi C, Selvi E, Iuliano A, Minisola G, Caporali R, Prisco E, Bombardieri S. A phase IB clinical trial with Dekavil (F8-IL10), an immunoregulatory 'armed antibody' for the treatment of rheumatoid arthritis, used in combination wiIh methotrexate. Isr Med Assoc J 2014; 16:666. [PubMed] [Google Scholar]
- 154.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000; 13:715–25 [DOI] [PubMed] [Google Scholar]
- 155.Sun L, He C, Nair L, Yeung J, Egwuagu CE. Interleukin 12 (IL-12) family cytokines: role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015; 75:249–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lasek W, Zagożdżon R, Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy?. Cancer Immunol Immunother 2014; 63:419–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L, Neri D. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol 2002; 20:264–9 [DOI] [PubMed] [Google Scholar]
- 158.Kim JW, Heery CR, Bilusic M, Singh NK, Madan RA, Sabzevari H, Schlom J, Gulley JL. First-in-human phase I trial of NHS-IL12 in advanced solid tumors. J Clin Oncol 2012; 30:TPS2617-TPS [Google Scholar]
- 159.Rudman SM, Jameson MB, McKeage MJ, Savage P, Jodrell DI, Harries M, Acton G, Erlandsson F, Spicer JF. A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res 2011; 17:1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol 2003; 111:677–90; quiz 91. [DOI] [PubMed] [Google Scholar]
- 161.Wynn TA. IL-13 Effector Functions. Annu Rev Immunol 2003; 21:425–56 [DOI] [PubMed] [Google Scholar]
- 162.Lebel-Binay S, Laguerre B, Quintin-Colonna F, Conjeaud H, Magazin M, Miloux B, Pecceu F, Caput D, Ferrara P, Fradelizi D. Experimental gene therapy of cancer using tumor cells engineered to secrete interleukin-13. Eur J Immunol 1995; 25:2340–8 [DOI] [PubMed] [Google Scholar]
- 163.Serve H, Oelmann E, Herweg A, Oberberg D, Serve S, Reufi B, Mucke C, Minty A, Thiel E, Berdel WE. Inhibition of proliferation and clonal growth of human breast cancer cells by interleukin 13. Cancer Res 1996; 56:3583–8 [PubMed] [Google Scholar]
- 164.Waldmann TA. Interleukin-15 in the treatment of cancer. Expert Rev Clin Immunol 2014; 10:1689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wege AK, Weber F, Kroemer A, Ortmann O, Nimmerjahn F, Brockhoff G. IL-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (HTM). Oncotarget 2017; 8:2731–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vincent M, Bessard A, Cochonneau D, Teppaz G, Sole V, Maillasson M, Birkle S, Garrigue-Antar L, Quemener A, Jacques Y. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. Int J Cancer 2013; 133:757–65 [DOI] [PubMed] [Google Scholar]
- 167.Vincent M, Quéméner A, Jacques Y. Antitumor activity of an immunocytokine composed of an anti-GD2 antibody and the IL-15 superagonist RLI. Oncoimmunology 2013; 2:e26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu Y, Zhong Z, Martomo S, Lu D, Polonskaya Z, Luna X, Zhang H, Zhang Z, Wang Z, Liu L, Patel J, Tonra J, Li H, Witte L, Waksal S, Zhu Z. Abstract C173: anti-PD-L1 antibody-based IL-15 immunocytokine has enhanced antitumor immunity. Mol Cancer Ther 2015; 14:C173–C. [Google Scholar]
- 169.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol 2002; 71:1–8 [PubMed] [Google Scholar]
- 170.Zrioual S, Toh ML, Tournadre A, Zhou Y, Cazalis MA, Pachot A, Miossec V, Miossec P. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J Immunol 2008; 180:655–63 [DOI] [PubMed] [Google Scholar]
- 171.Miossec P. Update on interleukin-17: a role in the pathogenesis of inflammatory arthritis and implication for clinical practice. RMD Open 2017; 3:e000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol 2009; 183:4169–75 [DOI] [PubMed] [Google Scholar]
- 173.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov 2014; 13:21–38 [DOI] [PubMed] [Google Scholar]
- 174.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev 2013; 252:116–32 [DOI] [PubMed] [Google Scholar]
- 175.Kim K, Kim G, Kim JY, Yun HJ, Lim SC, Choi HS. Interleukin-22 promotes epithelial cell transformation and breast tumorigenesis via MAP3K8 activation. Carcinogenesis 2014; 35:1352–61 [DOI] [PubMed] [Google Scholar]
- 176.Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, Harrison O, Powrie F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med 2013; 210:917–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007; 445:648–51 [DOI] [PubMed] [Google Scholar]
- 178.Shioya M, Andoh A, Kakinoki S, Nishida A, Fujiyama Y. Interleukin 22 receptor 1 expression in pancreas islets. Pancreas 2008; 36:197–9 [DOI] [PubMed] [Google Scholar]
- 179.Dudakov JA, Hanash AM, van den Brink MRM. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 2015; 33:747–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP, Das I, Wang R, Chen AC, Loudovaris T, Kay TW, Thomas HE, Whitehead JP, Forbes JM, Prins JB, McGuckin MA. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med 2014; 20:1417–26 [DOI] [PubMed] [Google Scholar]
- 181.Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, Diehl L, van Bruggen N, Kolumam G, Ouyang W. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014; 514:237–41 [DOI] [PubMed] [Google Scholar]
- 182.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008; 29:947–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ng S, Galipeau J. Concise review: engineering the fusion of cytokines for the modulation of immune cellular responses in cancer and autoimmune disorders. Stem Cells Transl Med 2015; 4:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li P, Yuan S, Galipeau J. A fusion cytokine coupling GMCSF to IL9 induces heterologous receptor clustering and STAT1 hyperactivation through JAK2 promiscuity. PLoS One 2013; 8:e69405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Williams P, Galipeau J. GMCSF-interleukin fusion cytokines induce novel immune effectors that can serve as biopharmaceuticals for treatment of autoimmunity and cancer. J Intern Med 2011; 269:74–84 [DOI] [PubMed] [Google Scholar]
- 186.Deng J, Pennati A, Cohen JB, Wu Y, Ng S, Wu JH, Flowers CR, Galipeau J. GIFT4 fusokine converts leukemic B cells into immune helper cells. J Transl Med 2016; 14:106. [DOI] [PMC free article] [PubMed] [Google Scholar]