Figure 7.
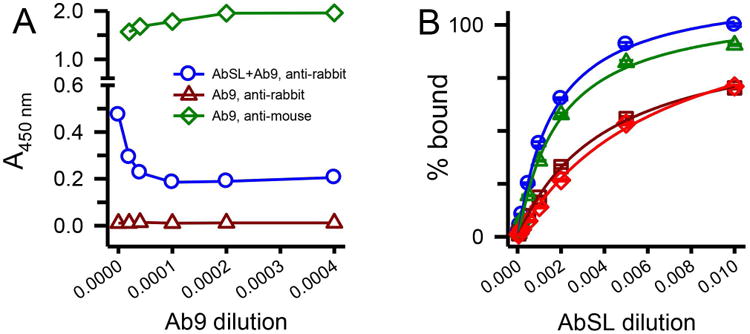
An N-terminal Aβ antibody partially hinders AbSL binding to protofibrils. Panel A. ELISA plates were coated with SEC-isolated Aβ protofibrils (18 ng/well) and treated with premixed primary antibody solutions containing AbSL (1:1,000) and varying dilutions of Ab9 (2,500-50,000; 0.1-2 μg/mL). Ab9 dilutions are represented on the x-axis as the inverse value of the dilution. Detection of primary antibodies AbSL and Ab9 was done with secondary antibodies anti-rabbit IgG-HRP (for AbSL, blue circles) or anti-mouse IgG-HRP (for Ab9, green diamonds). No detection of Ab9 was observed when anti-rabbit IgG-HRP was used as the secondary antibody (dark red triangles). Panel B. Immunoplates were coated with Aβ protofibrils (18 ng/well) and treated with premixed primary antibody solutions. These solutions contained increasing dilutions of AbSL (100, 500, 1,000, 2,000, 5,000, 10,000, and 20,000) either in the absence (blue circles) or presence of constant amounts of Ab5. Repeated experiments were done at constant Ab5 dilutions of 1:10,000 (green triangles), 1:2,000 (dark red squares), and 1:1,000 (red diamonds). Each experiment determined AbSL binding in the absence of Ab5. Data points (± SEM) represent the average of n=3 trials for each Ab5 dilution and n=12 trials for AbSL alone. Antibody binding on the y-axis is presented as percent A450 of the highest AbSL concentration (1:100) in the absence of Ab5.
