Abstract
Hydrogels are promising biomaterials because of their important qualities such as biocompatibility, biodegradability, hydrophilicity and non-toxicity. These qualities make hydrogels suitable for application in medical and pharmaceutical field. Recently, a tremendous growth of hydrogel application is seen, especially as gel and patch form, in transdermal drug delivery. This review mainly focuses on the types of hydrogels based on cross-linking and; secondly to describe the possible synthesis methods to design hydrogels for different pharmaceutical applications. The synthesis and chemistry of these hydrogels are discussed using specific pharmaceutical examples. The structure and water content in a typical hydrogel have also been discussed.
Keywords: Hydrogel, Cross-linking, Thermoreversible gel, Polymers
Introduction
The formulations applied onto the skin surface are broadly classified in to two groups such as topical and transdermal. Topical formulations deliver drug to local area of skin without systemic exposure. On the other hand, transdermal formulations applied to the skin surface for the purpose of delivering and maintaining effective concentration of drug in the systemic circulation.1 There are three basic types of transdermal formulations such as aerosol sprays, semisolids, and patches. Out of these, semisolid transdermal drug formulations such as creams, ointments, and gels are most commonly used to provide systemic effect by delivering the drug across the skin. Gel, among all, is most preferred because of their excellent appearance, fast drug release, desired consistency, ease of manufacturing and quality assessment, and considerable stability.2 Most importantly, gels can be modified in order to make it suitable for delivering drug in sustained manner.
An ideal gel should satisfy the following three salient features:3
They must have at least two components i.e. the gelling agent and a fluid component.
Each component should be continuous throughout the system.
They should exhibit mechanical properties of the solid state.
Gels are transparent or translucent semisolid formulations containing a high ratio of solvent/ gelling agent. In other word, gel can be defined as a semi-solid preparation composed of low concentrations (<15%) of gelator molecules.4 According to USP, gels are defined as a semisolid, either suspension of small inorganic particles or large organic molecules interpenetrated with liquid.5 In gel system a liquid phase is constrained within a rigid three dimensional polymeric network thereby exhibiting visco-elastic nature. Gels can be classified into hydrogels and organogels based on the liquid medium entrapped within the polymeric network. Hydrogels are composed of aqueous phase, whereas organogels are composed of aqueous phase along with organic phase.
Hydrogels are hydrophilic, three dimensional cross-linked polymer systems capable of imbibing large amounts of water or biological fluids between their polymeric chains to form aqueous semi-solid/solid gel networks.6,7 Polymer networks in hydrogel may absorb water from 10–20% (an arbitrary lower limit) up to thousands of times their dry weight.8,9
Followings are the main features of hydrogels for which they are widely used in pharmaceutical applications:10-14
Both compositionally (such as glycosaminoglycans) and mechanically, hydrogels are similar to the native extracellular matrix, thus can serve as a supporting material for cells during tissue regeneration and carrier in delivering a therapeutic agent.
It exhibits soft material nature, which encourages uptake of water and thereby forming hydrated yet solid materials, just like cells in the body.
The elastic property (due to the presence of meshes of the networks) of fully swollen or hydrated hydrogels is found to be minimizing irritation to the surrounding tissues after implantation.
Their hydrophilic and cross-linked property imparts excellent biocompatibility.
Low interfacial tension between the hydrogel surface and body fluid minimizes protein adsorption and cell adhesion, which reduces the chances of a negative immune reaction.
Mucoadhesive and bioadhesive characteristics of many polymers used in hydrogel preparations (e.g. polyacrylic acid (PAA), polyethylene glycol (PEG), and polyvinyl alcohol (PVA)) enhance drug residence time on the skin/plasma membrane, leading to increase in tissue permeability.
Structure and water content in hydrogel
Presence of water in hydrogels plays an important role in the overall permeation of active ingredients into and out of the gel. Water can be associated to any hydrogel structure in following ways as shown in Figure 1.
Figure 1.
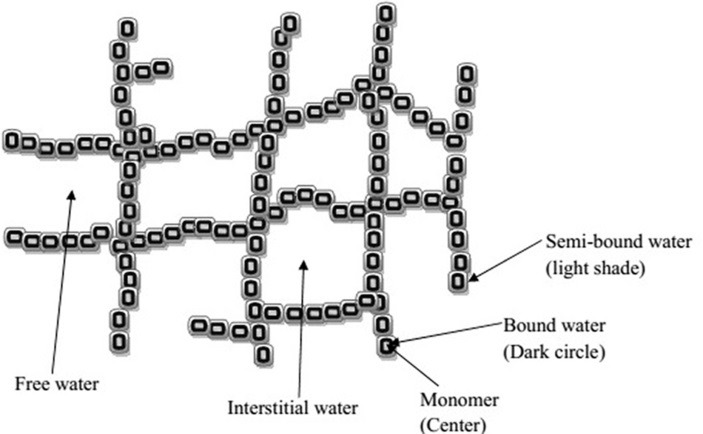
Schematic diagram of molecular structure of hydrogel network with different types of water.
The types of water associated with hydrogel are:
Bound water (primary and secondary)
Semibound water
Interstitial water
Free water or bulk water
When a dry hydrogel comes in contact with water, absorption of water into its matrix starts. At first water molecules entering the matrix will directly attach to the hydrophilic groups, forming hydrophilic bound water or primary bound water. The polymeric network swells because of complete hydration of the polar groups that resulted in the exposer of hydrophobic groups. These exposed groups also interact with water molecules, leading to hydrophobically-bound water, or ‘secondary bound water’. The combination of primary and secondary bound water is often called the bound water. This water is considered as integral part of hydrogel structure and can only be separated out from the hydrogel under extreme conditions. After the saturation of hydrophilic and hydrophobic groups, the additional water that is absorbed due to the osmotic driving force of the network chains is called free water or bulk water. Between the bound water, present on polymeric monomer surface, and free water, there is a presence of water layer called semibound water. Interstitial water is present in the interstices of hydrated polymeric network that is trapped physically but not attached to hydrogel network.7,15
Classification of hydrogel
Pharmaceutical hydrogels may be classified on the basis of their type of cross-linking or microstructure into following types (Figure 2):
Figure 2.
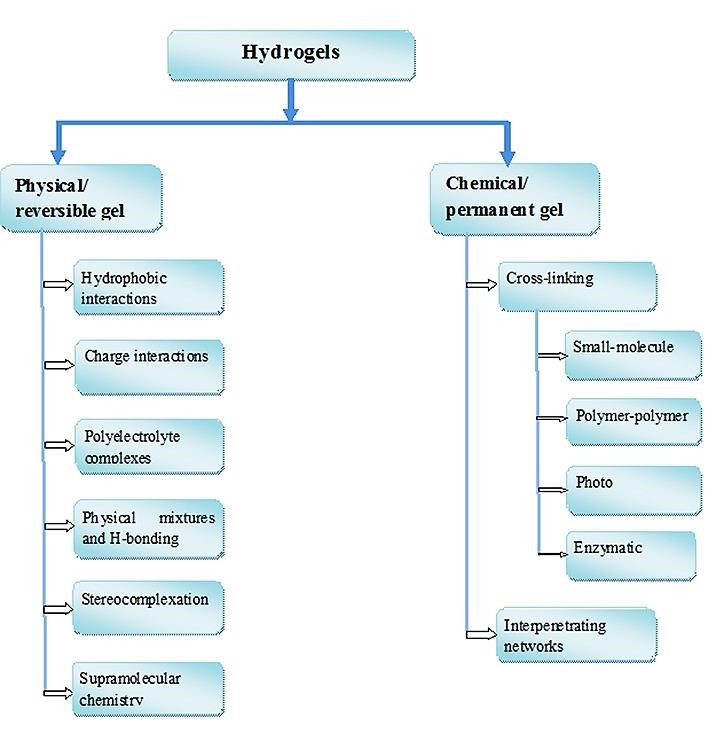
Classification of hydrogels based on cross-linking
Physical or reversible gel
In the preparation of physical gel, the selection of polymer is crucial and it depends on the two primary criteria; interaction among the chain must be strong enough to form semi-permanent junction in the molecular network and the network should hold large amount of water molecules inside it. The forces involved in the physical gel formation are hydrophobic, electrostatic, and hydrogen bonding between polymer chains.16,17 Because the network formation by all of these interactions is purely physical, gel formation can be reversed.
Hydrophobic interactions
The mechanism involved in the sol-gel and gel-sol conversion of thermoreversible gel is based on hydrophobic interaction. The polymers showing hydrophobic interaction must be having both hydrophilic and hydrophobic domain, called as amphiphiles. Such amphiphiles are water soluble at low temperature. However, following increased temperature the hydrophobic domains (gelators) aggregate to minimize the hydrophobic surface area contacting the bulk water. This reduces the amount of structured water surrounding the hydrophobic domains and maximizing the solvent entropy. The gelation temperature depends on various parameters such as the concentration of the polymer, the length of the hydrophobic block, and the chemical structure of the polymer.18
Poloxamer (PX) is such a polymer which shows thermoreversible property in aqueous solutions. PX consists of hydrophilic poly (ethylene oxide) (PEO) and hydrophobic poly(propylene oxide) (PPO) blocks arranged in a tri-block structure as PEO–PPO–PEO [19]. The gelation of PX depends upon both temperature and concentration and the total gelling process is typically divided into two steps (Figure 3). The first step occurs when the temperature is increased to reach the critical micelle temperature (CMT) and the PX co-polymers aggregate to form spherical micelles. These micelles consist of an outer shell of hydrated swollen PEO chains with dehydrated PPO blocks as the core. The process develops into the second step when a further increase in the temperature packs the micelles in an orderly manner to form gels.19 Gelation is also dependent on the concentration of PX molecules in solution. At very low concentrations, PX molecules exist as monomers in solution. Increasing PX concentration up to 10−4% to 10−5% (w/w) leads to a critical micelle concentration (CMC), where spherical micelles started to built up. Further increase in PX concentration leads to a tightly packed system with a gel consistency.20,21 Between 20-30% (w/v) aqueous solution of PX 407 at cold temperature (4-5°C) forms clear liquid and get transformed to gel at body temperature (37°C).
Figure 3.
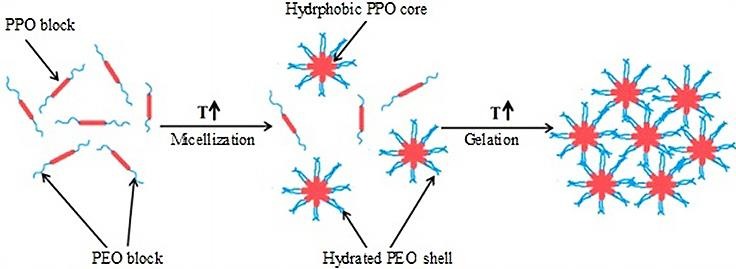
Schematic representation of physical gelation mechanism of PX 407 in water (Modified from reference 20)
Release of the model drug benzoic acid was found to be decreased with the increase in PX 407 concentration. This is probably due to an increase in size and number of micelles leading to decrease in size and number of aqueous channels. Further the modification of benzoic acid to more lipophilic moiety p-hydroxybenzoic acid causes a decrease in its release rate. This could be due to the greater partitioning of lipophilic derivatives into the micellar region within the gel structure.22 Erukova et al. (2000) observed that PX facilitated the permeation of comparatively large molecules, including 2-n-undecylmalonic acid and doxorubicin, across lipid bilayers, whereas the permeation of small solutes (such as ammonium and acetic acid) remained unaffected. They also observed that PX accelerated the translocation of large hydrophobic anions (tetraphenylborate). They attributed the above effect of PX with the content of PEO units i.e. it is enhanced when the portion of PEO block in the copolymer is increased.23 Gels and niosomal gels of meloxicam were developed using polymers such as PX 407, Chitosan and Carbopol-934. The PX 407 gel or niosomal PX 407 gel showed the superior drug release over the other formulations. Between the two types of gel of PX 407, niosomal gels exhibited superiority over conventional gels for anti-inflammatory test.24 Thermosensitive gels of drugs such as indomethacin,25 adriamycin and 5-flurouracil,26 mitomycin C,27 and ketoprofen28 were successfully developed and studied. The gel formed from PX alone (as in case of above) leads to relatively rapid diffusion of drugs out of the hydrophilic gel matrix by the dilution of water that entered the gel matrix.29 To circumvent above drawback many researchers have used combination of polymers to reduce the fast entry of water and subsequently diffusion of drug out of gel structure. In addition, it will retain the gel structure for longer period of time and thereby sustain the drug release.
Based on the above fact thermoreversible gels of meloxicam were prepared using PX 407 and PX 188 (20-30% w/w) in combination with different additives such polyvinylmethylether maleic anhydride copolymer, HPMC, PE-400, DMSO, sodium chloride. Among investigated gel bases, PX 407-HPMC gel was found to be ideal due to its gel strength (1.560±0.0135 N), viscosity (312.3±2.06 cP) and release characteristics.30 HPMC K100M (2% w/v) and PX 407 (20% w/v) based procaine bioadhesive gels were prepared and the drug release was found to be increased with the increase in drug concentration from 1.5% to 3.5% (w/w) in the gels and the temperature of surrounding solutions from 28°C to 42°C.31 Pranoprofen release from HPMC and PX 407 based bioadhesive gel was increased with the increase in temperature, which was attributed to increased in activation energy of the drug.32,33 Similarly, Shin et al. (2004) successfully prepared lidocaine HCl gel using HPMC (2% w/v) and PX 407 (20% w/v) for analgesic activity.34 We have successfully prepared and characterized bioadhesive hydrogels of aceclofenac and metoprolol succinate containing various concentrations of HPMC (0.5-4% w/w) and PX 407 (15-30% w/w).35,36
A range of other synthetic amphiphilic thermally gelling polymers have been investigated. Based on the position of hydrophilic and hydrophobic blocks they may be classified in to two categories; symmetric and asymmetric. Symmetric means the presence of hydrophobic block at the center and hydrophilic blocks on either side and vice versa. Examples of this category are PX (discussed in the previous section), PEG- poly (lactide-co-glycolide) (PLGA)-PEG37 or PLGA-PEG-PLGA38 etc. Compared to PX systems, PL(G)A-based triblock gelators exhibit better biodegradability, higher gelation temperatures (which is allowing easier handling of injectable preparation), and sustained drug release.39 Another example of symmetric triblock polymer is Poly (N-isopropylacrylamide) (PNIPAM)-poly (phosphorylcholine)-PNIPAM, which form gels at 6-7 wt % when the temperature exceeded 32°C (is the phase transition temperature of PNIPAM).40 PNIPAM grafted with other polymers such as hyaluronic acid and chitosan were developed successfully for the sustained release of riboflavin41 and 5-fluorouracil,42 respectively. Asymmetric triblock copolymer formed of PEG, PLA, and poly (L-glutamic acid) were also developed and observed that the L-glutamic acid block permits selective modification with specific targeting groups such as the cell-adhesive RGD peptide.43
Reverse thermal gelation is also evident from some natural polymers. A biocompatible, parenteral gel composed of chitosan solutions and glycerol-2-phosphate was prepared and the gel was found to be exhibited sol-gel transition at a temperature close to 37°C.44 Chitosan grafted with PEG (approximately 40 wt %) based thermoreversible gel was prepared and studied for bovine serum albumin (BSA) release. This gel showed an initial burst release for 5 h and afterwards a steady linear release of BSA for a period of approximately 70 h.45 Liang et al. (2004) successfully used thermosensitive methylcellulose to thermally gel aqueous alginate solution blended with various salts such as CaCl2, Na2HPO4, and NaCl for the delivery of BSA.46 Similar thermally triggered transitions have also been exhibited by hydroxypropylcellulose.47
Charge interactions
Charge interactions which lead to the formation of hydrogel may occur in two ways; (i) between a polymer and an oppositely charged small molecule as linker, and (ii) between two polymers of opposite charge (Figure 4). This type of cross-linking (or decross-linking) can be triggered by pH changes that ionize or protonate the ionic functional groups. These hydrogels can be tailored to achieve a specific set of thermal, mechanical and degradation profiles. In some cases they can formed in situ, known as in situ gelling hydrogels.48 A mixture of quaternized chitosan (N-[(2-hydroxy-3-trimethylammonium) propyl] chitosan chloride (HTCC)) and glycerophosphate (GP) forms an ionically cross-linked gel at 37°C which can release doxorubicin hydrochloride as a function of pH.49 At acidic condition and physiological condition these hydrogel dissolved and released drug quickly, while it released drug slowly at neutral or basic conditions.
Figure 4.
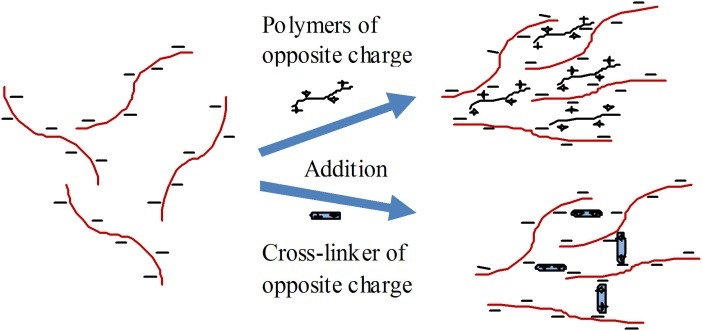
Schematic illustration of mechanism of charge interactions with an oppositely charged polymer and a cross-linker
The charged interactions have been widely investigated for cross-linking in situ gelling hydrogels. The main advantage of this interaction is that biodegradation can occur as ionic species in extracellular fluid which bind competitively with the gel components and as a result decross-linking of network happen. In addition, this approach can also be used to cross-link microparticle or nanoparticle gels to equip it with suitable drug delivery properties. For instance anionic (methacrylic acid (MAA)) and cationic (dimethylaminoethyl methacrylate (DMAEMA)) polymers coated hydroxyethyl methacrylate-derivatized dextran microspheres exhibit spontaneous gelation upon mixing due to ionic complex formation between the oppositely charged microparticles.50 Another approach, so called ionic-complementarity, uses peptide of alternating positive and negative charge distribution leading to peptide self-assembly. These peptides assume β-sheet secondary structure predominantly, and can form hydrogels. The advantage of this approach is that nanoscopic and/or macroscopic structures with great stability and functionality can be developed by varying peptide concentration, pH, presence of salts, and time.51
Polyelectrolyte complexes (PECs)/ Complex coacervate gel
PECs are formed by the electrostatic interactions between two oppositely charged polyelectrolytes in an aqueous solution as shown as in Figure 5. Thus both the polymers must be ionized and have opposite charges. This implies that the reaction can only occur at pH values in the vicinity of the pKa interval of the two polymers.16 Its distinction from electrostatic interactions is based on the MW of molecules. The PEC is formed between larger molecules with a broad MW range and the association is stronger than other secondary binding interactions like hydrogen bonding (H-bonding) or van der Waals interactions. For example formation of PEC between chitosan, a polycation, with polyanions such as proteins (e.g. collagen, gelatin, albumin), polysaccharides (e.g. alginate, pectin or xanthan) and synthetic polymers (e.g. PAA) were reported.52
Figure 5.
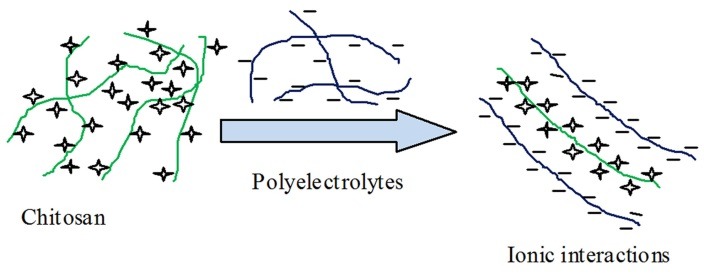
Schematic diagram of PEC mechanism
Chitosan based PEC of metoprolol tartrate were developed in the form of beads and hydrogels by interaction of cationic chitosan with anionic sodium alginate, carboxymethyl cellulose sodium and k-carrageenan using simple ionic gelation technique. Among all the combinations, PEC of chitosan and carboxymethylcellulose sodium exhibited least in vitro drug release and was able to extend the release of metoprolol tartrate up to 12 h.53 Another chitosan based PEC hydrogel with improved viscoe-lastic behavior was developed using low concentration (0.1 to 0.8%) of chitosan and constant gelatin concentration of 1%.54 Chang et al. (2014) successfully developed PEC of chitosan and poly-(γ-glutamic acid) (γ-PGA) to promote new bone formation in the alveolar socket following tooth extraction.55 With same composition, a cytocompatible PEC was developed for biological application.56 A PEC composed of alginate and chitosan was developed and found to be having improved thermal, chemical, and mechanical properties and stability.57 Sometimes, same polymers having opposite charges, fraction is modified to opposite charge by chemical processes, can also be used to prepare PEC hydrogel. One of such hydrogel was developed with phosphorylated chitosan (a polycation) and chitosan (a polyanion) as an osteoblast carrier. These hydrogels exhibited three-dimensional hierarchically-porous structure and good cyto-biocompatibility for osteoblasts in vitro.58 Based on above approach, another novel PEC encapsulated dexamethasone hydrogel was prepared using polycationic N-trimethyl chitosan and polyanionic N-carboxymethyl chitosan to target distal intestine.59 Chitosan, in many drug delivery systems, present as unionized amine form at the neutral/alkaline pH of physiological application sites, while the anionic polyelectrolyte is in the ionized form. In this form to stabilize any PEC is a challenging task, but it can be overcome by using modified chitosan such as quaternized chitosan. This modified chitosan along with PAA were used to prepare PEC hydrogels with improved mechanical properties by in situ polymerization of acrylic acid monomers in the concentrated quaternized chitosan solution.60
The advantage of PEC is that it is formed without the use of organic precursors, catalysts, or reactive agents, avoiding the concern about safety in the body. During complexation, polyelectrolytes can either coacervate, or form a more or less compact hydrogel. However, if ionic interactions are too strong, precipitation can occur,61 which is quite common and hinders the formation of hydrogels. Precipitation can be prevented weakening the electrostatic attraction by the addition of salts, such as NaCl.16
Physical mixtures and hydrogen bonding interaction
Hydrogels by H-bonding interactions can be formed by freeze-thaw cycles using PVA alone62 or in combination with other polymers such as chitosan.52 In the earlier case crystallization plays a big part in the creation of cross-links between PVA chains during lyophilization. The cross-linking is possible due to the existence of regular pendant hydroxyl groups on PVA that are able to form crystallites by strong inter-chain H-bonding. The crystals formed by this method are generally considered to have a two-molecule monoclinic unit cell, with hydroxyl groups randomly placed on either side of both polymer chains as shown in Figure 6. It has been proposed that in the first freeze-thaw cycle, the initial freezing of water, and associated expansion, leads to increased polymer concentration in the unfrozen phase. Crystallization can then take place within the polymer-rich microphages.63
Figure 6.
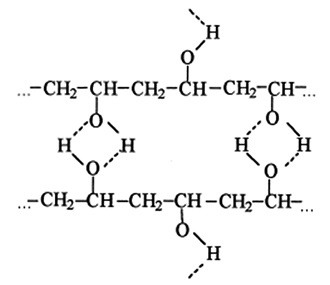
Hydrogen bonding in PVA solutions
Processing parameters such as the concentration, the number of thermal cycles, and the thawing rate affect the mechanical behavior of cryogel. Pazos et al. (2009) reported that with increasing number of thermal cycles resulting in the formation of the strongest cryogels because the number of intermolecular hydrogen bonding increases and molecular chains aggregate.64 They also reported that the increase in thawing rate from 2 (0.333°C/min) to 5 (0.067°C/min) exhibited the same effect on the gel’s elastic properties. Meenakshi, and Ahuja, (2015) found that 4 freeze-thaw cycles are optimum for the preparation of composite cryogels of metronidazole with carboxymethyl tamarind kernel polysaccharide and PVA. This cryogel provided 75.77% of drug release over a period of 6h and had better thermal stability.65
In case of composite cryogel, mixture of polymers forms junction points in the form of crystallites and inter-polymer interaction after a series of freeze–thaw cycles.16 These interactions act as cross-linking sites of the hydrogel. Addition of another polymer to PVA negatively affects the formation of PVA crystallites thereby forming hydrogels of less ordered structures. Chitosan-PVA polymer blend is one such example of polymer mixture, where increasing chitosan concentration in the blend reduces the crystallinity of hydrogel.52 The addition of chitosan decreased maximum strength and thermal stability of PVA cryogel containing minocycline and gentamycin, while it increased the swelling ability, elasticity and porosity of cryogel.66,67 Contrary to above, Paduraru et al. (2012) reported that strength of the cryogel increased by increasing the microcrystalline cellulose content from 0 to 50 % wt.68
H-bonding can also be formed with blend of two or more natural polymers such as hyaluronic acid-methylcellulose, gelatin-agar, and starch-carboxymethyl cellulose. The formation of H-bonding interactions between the polymer chains along with the compatible geometry resulted in the enhanced visco-elastic properties of the hydrogel.69 The main advantage of above mixture of polymers is the excellent biocompatibility offered by them due to the absence of chemical cross-linkers. However, the problem associated with it is the breaking of H-bonded networks over a few hours in vivo due to influx of water, which restricting their use to short-acting drug release systems.18
Stereocomplexation
Stereocomplexation is a synergistic interaction between polymer chains or small molecules of the same chemical composition, occasionally with different chemical composition, but different stereochemistry and stereocomplex formation occur due to the interaction between polymers having different tacticities or configurations.70 It may be homo-stereocomplexation or hetero-stereocomplexation. In former case the complex formation occurs between same molecules but with different stereochemistry. For example, interaction between polylactide blocks with L- and D-stereochemistry.70 Another well known example of stereocomplexation is the one between isotactic and syndiotactic poly(methyl methacrylate) (PMMA).71 Hetero-stereocomplexation occurs between two different molecules with different stereochemistry such as D-PLA and L-configured peptides leuproide, a luteinizing hormone-releasing hormone.72 Natural polymers such as dextran precursors can be grafted to L-lactide and D-lactide oligomers leading to the spontaneous gelation in water. It provided an excellent biocompatibility and biodegradability.73
Like H-bond interactions, stereocomplexation do not need harsh organic solvents and chemical cross-linkers. Main limitation of stereocomplexation is the restricted range of polymer compositions available which can be used i.e. even small changes in the stereochemistry or else changing the composition of matter can significantly weaken or altogether eliminate the stereochemical interaction.
Supramolecular chemistry
Supramolecular chemistry is based on the association of two or more chemical species held together by intermolecular forces. The intermolecular forces includes electrostatic interactions, H-bonding, metal coordination, π- π interaction, Van der Waals forces etc. and the important methods by which supermolecules formed are; molecular self-assembly, folding, host-guest interaction (e.g. inclusion complex), recognization and complexation and mechanical-interlocking.74
This approach has wide application such as material technology, catalysis, data storage and processing and in the preparation of medicines. The most common type of cross-linking interaction used to prepare hydrogels is the formation of inclusion complexes. For example, formation of a reversible hydrogel complex between PEO polymers and α-cyclodextrins.75 Similarly, β-cyclodextrin can be used to gel PPO-grafted dextran into a hydrogel.76 Self-assembled systems have also been reported that apply both hydrophobic interactions and supermolecular chemistry to facilitate the formation of denser and/or more stable hydrogel networks. A self-assembled system, PEO-poly(R-3-hydroxybutyrate) (PHB)-PEO triblock copolymers, was complexed with α-cyclodextrin to form strong hydrogel networks. The cross-linking was accomplished by (a) the thermal gelation of the hydrophobic PHB segments and (b) the inclusion complexes formed between the PEO segments and cyclodextrin. This system is capable of releasing fluorescein isothiocyanate-dextran for up to one month.77
Chemical or permanent gel
Physically cross-linked hydrogels are prepared without the use of cross-linking entities or chemical modification. Despite of the above advantage, it is inflexible towards variables such as gelation time, gel pore size, chemical functionalization, and degradation or dissolution, leading to inconsistent performance in vivo.52 In contrast, chemically cross-linked hydrogels allows absorption of water and/or bioactive compounds without dissolution and permits drug release by diffusion.18
Cross-linking
Chemical cross-linking method uses covalent bonding between polymer chains in order to produce permanent hydrogel. The cross-link formation was carried out by the addition of small cross-linkers molecules, polymer-polymer conjugation, photosensitive agents or by enzyme catalyzed reaction.
Small-molecule cross-linking
Preparation of small-molecule cross linking hydrogels at the minimum requires one polymer and a small molecule as cross-linker in an appropriate solvent. Cross-linkers are molecules with at least two reactive functional groups that allow the formation of bridges between polymeric chains.78 Small cross-linkers can be of two types; bi-functional molecules or drug molecules. In the former case drug molecule is entrapped within the hydrogel formed due to the interaction between bi-functional molecule and polymer. Moreover, drug molecule having two functional group covalently bonded with polymeric chains to form hydrogel, which avoids the use of cross-linkers. This approach is limited to drug in which two reactive functional groups are available. One such drug molecule is primaquine (a di-amino drug), which has been used to cross-link periodate-oxidized gum arabic into a hydrogel through the rapid formation of Schiff bases between the amine groups and the aldehyde groups present in the drug and polymer, respectively.79
The simplest form of the cross-linking takes place between aldehyde and amino groups to form Schiff base. For example, dialdehyde such as glyoxal and particularly glutaraldehyde46 forms covalent imine bonds with the amino groups of chitosan via Schiff reaction.80 In other cases, dextran-tyramine81 and hyaluronic acid-tyramine82 covalently bonded by using horseradish peroxidase (HRP) and hydrogen peroxide (H2O2) as cross-linkers to form hydrogels with improved controllable gelation times ranging from 5 s to 9 min according to the reactant concentrations used. A general disadvantage of each of these small-molecule cross-linking methods is the potential toxicity of residual unreacted cross-linker agents in vivo. For instance, glutaraldehyde and glyoxal are known to be neurotoxic and mutagenic, respectively.80
Genipin (a naturally derived chemical from the fruit ofgardenia) widely used as a cross-linking agent alternative to dialdehydes due to its biocompatibility.83 It was also reported that genipin bind polymers, such as chitosan and gelatin with biological tissues covalently.84 Furthermore, amino-terminated groups containing molecules such as PEG, N,O-carboxymethyl chitosan,85 and BSA86 have been cross-linked to genipin to form hydrogels with flexible dissolution rates from 3 min to more than 100 days. Even though genipin shows good biocompatibility, it is still liable to negatively interact with encapsulated drugs, an unavoidable problem for gelation in the presence of a therapeutic.
This approach used to develop transdermal drug delivery systems for the delivery of oxprenolol HCl,87 propanolol HCl,88 5-fluorouracil,89 indomethacin from gel beads for the control release.90
Polymer-polymer cross-linking or hybrid polymer networks (HPN)
In this type of hydrogel, the cross-linking reaction occurs between a structural unit of a polymeric chain and a structural unit of another polymeric chain of another type. Therefore, polymers must be pre-functionalized with reactive functional groups. Several types of covalent linkages can be made depending on the desired speed of cross-linking, selection of targeted reactive functional groups, and biodegradability of the resulting conjugates.
Widely investigated in situ cross-linking phenomenon is Michael addition between a nucleophile (i.e. an amine or a thiol) and a vinyl group. For example, cross-linking of vinyl sulfone-functionalized dextrans with thiolated PEG. This type of approach provides the flexibility in forming multiple types of bonds, relative biological inertness and finally rapid formation of hydrogel.91 Another example is the formation of a hydrazone bond between an aldehyde and a hydrazide which facilitates rapid cross-linking of gel precursors.92 Control release of tissue plasminogen activator and budesonide93 to the peritoneum was achieved by using hyaluronic acid cross-linking by hydrazone bonds. Similar approaches also been used to design poly (aldehyde guluronate) for the effective delivery of osteoblasts and growth factors.94
The major advantages of this approach are the elimination of cross-linker molecules and in situ formation of covalently bonded hydrogels. The main limitation of this approach is the requirement for significant polymeric modification to link functional group to polymeric chain. In addition, the pre-gel polymers are often themselves somewhat cytotoxic, even when prepared from highly biocompatible polymer precursors.
Photo cross-linking
Formation of hydrogels based on photo cross-linking depends on the presence of photo sensitive functional groups. By linking a photo sensitive functional group to a polymer enables it to form cross-linkages upon irradiation with light such as UV light. Chitosan is one such polymer which was studied more compared to other polymers. A photo cross-linked chitosan hydrogel was developed by incorporating azide groups (-N3) to polymeric chain of chitosan. After its exposure to UV light, the azide group is converted to nitrene group (R-N:) which binds free amino groups of chitosan leading to the in situ formation of hydrogel within a minute.95 Photo cross-linked hydrogel can also be formed between the polymers. One such example is thermo-sensitive chitosan-pluronic hydrogel, where both the polymers were functionalized with photo sensitive acrylate groups (CH2=CHCOO−) by UV irradiation. The resulted cross-linked polymer formed a physical network at temperatures above the lower critical solution temperature (LCST) and the same exhibited the ability to release encapsulated human growth hormone (hGH) in sustained manner.96 The above combination was also used to deliver plasmid DNA.97 Another chitosan-PEG-based hydrogel was developed by modifying chitosan with photoreactive azidobenzoic acid and PEG with arginylglycylaspartic acid peptide. Upon UV illumination, a free-radical photo-initiated polymerization took place that led to the formation of hydrogel in situ. Resulted hydrogel helped in targeting the injured myocardium for the delivery of growth factors and cells.98
The advantage of this approach is to facilitate easy and speedy formation of hydrogel. It also offers safety and low cost preparation compared to chemical methods, which generally require the addition of different reactive species, initiators, or catalysts. However, this technique requires a photosensitizer and prolonged irradiation, which could also result in local rise of temperature, thereby damaging neighboring cells and tissue.99
Enzymatic cross-linking
This is a new approach to form in situ hydrogel using enzyme-catalyzed cross-linking reaction between polymer chains. A number of enzymes including transglutaminases (TG),100-102 peroxidases,103,104tyrosinase,105 phosphopantetheinyl transferase, lysyl oxidase, plasma amine oxidase, and phosphatases106 were studied and reported.
TG belong to thiol group of enzymes which catalyze the formation of highly resistant covalent bonds between a free amine group of a protein or peptide-bound lysine and the g-carboxamide group of a protein or peptide-bound glutamine.106 Yung et al. (2007) have successfully developed thermally stable and biocompatible gelatin hydrogels cross-linked by microbial TG (mTG), which is capable of delivering encapsulated regenerative cells (HEK293) in a controlled manner.100 The same group tested the diffusion of anticancer drug, interleukin-2, from mTG cross-linked gelatin and found that the resulted hydrogels are not only cyto-compatible but also have potential to be used as sustained drug release devices.101 Between animal derived tissue TG (tTG) and recombinant human TG (hTG) enzymes used for cross-linking of two classes of protein polymers containing either lysine or glutamine under physiological conditions, tTG completed the cross-linking faster (within 2 min) compared to hTG.102
HRP and soy bean peroxidase are the most commonly used peroxidase enzymes in the formation of hydrogel. They catalyze the conjugation of phenol and aniline derivatives in the presence of substrate H2O2. In this reaction the HRP promptly combines with H2O2 and the resulted complex can oxidize hydroxyphenyl groups present in compound such as tyramine and tyrosine.106 Recently, Kim et al. (2011) developed HRP catalyzed injectable tyramine modified hyaluronic acid (HA-Tyr) hydrogels in two steps. In the first step, HA-Tyr conjugate was synthesized by amide bond formation between carboxyl groups of HA and amine groups of tyramine and in the subsequent step HA-Tyr hydrogels were prepared by radical cross-linking reaction using HRP and H2O2. This hydrogel was used to deliver dexamethasone intra-articularly for the treatment of rheumatoid arthritis.103 An enzymatically cross-linked injectable hydrogel was developed from chitosan derivatives, chitosan-glycolic acid, and phloretic acid using HRP and H2O2.104 This hydrogel can be formed (gelation time) from 10 sec to 4 min by using the polymer concentration from 3 to 1% and has potential for cartilage tissue engineering.
Tyrosinases are oxidative enzymes that convert accessible tyrosine residues of proteins (e.g. gelatin) into reactive o-quinone moiety, which can undergo non-enzymatic reactions with available nucleophiles such amino groups of chitosan. Chen et al. (2002) developed gelatin gel by two methods namely; (i) cooling the gelatin solution and (ii) tyrosinase-catalyzed hydrogel formation and observed that the second method demonstrated better mechanical properties which can be exploited for medical and industrial applications.107 Gelatin-chitosan gels were developed using both TG and tyrosinase for comparison and following observations were made; (i) chitosan was not required for transglutaminase-catalyzed gel formation, although gel formation was faster and the resulted gel was stronger in its presence, (ii) TG-catalyzed gelatin-chitosan gels lost the ability to undergo thermally reversible transitions, (iii) tyrosinase-catalyzed gelatin-chitosan gels were considerably weaker than transglutaminase-catalyzed gels, but can be strengthened by cooling below gel-point of gelatin.105
Interpenetrating networks (IPNs)
An IPN may be defined as any material that contains two or more polymers in the network form in which one polymer is cross-linked in the presence of other.108,109 IPNs are considered as “alloys” of cross-linked polymers, which are formed without any covalent bonds between them. These networks cannot be separated unless chemical bonds are broken.110
The polymers to be used in the preparation of IPN hydrogel must fulfill following three conditions; at least one polymer must be synthesized and/or cross-linked within the immediate presence of the other, both polymers should have similar kinetics, and there should not be dramatic phase separation between/among the polymers.111 An IPN is different from other polymer combination because it has no viscoelastic property, and it swells, but does not dissolve in any solvent.112
Based on the chemistry of preparation, IPN hydrogels can be classified into following types:113,114
Simultaneous IPN: when both the networks are synthesized simultaneously from the precursors at the same time by independent, non-interfering routs.
Sequential IPN: it is formed by swelling of a single-network hydrogel into a solution containing the mixture of monomer, initiator and activator, with or without a cross-linker.
Sequential IPNs are of two types namely, fully-IPN and semi-IPN based on the presence and absence of cross-linker, respectively.
According to the structure, IPN hydrogels can be classified into three categories:114
IPNs: formed by two juxtaposed networks, with many entanglements and physical interactions between them.
Homo-IPNs: these are developed from the two polymers which are having independent networks, but have same structure.
Semi- or Pseudo-IPNs: in this type one polymer has a linear instead of a network structure.
There are mainly two classes of polymers which are used to synthesize IPN hydrogels, including natural polymers and their derivatives such as polysaccharides and proteins, and synthetic polymers containing hydrophilic functional groups (e.g. -COOH, -OH, -CONH2, SO3H, amines etc).8
IPN hydrogels of clarithromycin were synthesized by using three polymers such as chitosan, PAA and poly (vinyl pyrrolidone) (PVP) and two cross-linking agents (e.g. glutaraldehyde and N,N’-methylene-bis-acrylamide). The resulted IPN hydrogels have good mucoadhesion property, which helped the gel to retain in gastric environment of stomach for prolonged period of time. As a result of that IPN hydrogel maintain antibiotic concentration in stomach for prolonged period of time, thereby used as a drug delivery system for treatment of H. pylori infection and in management of peptic ulcer.115 Semi-IPN hydrogels were synthesized by cross-linking chitosan and PVP blend with glutaraldehyde and found that the resulted semi-IPN gels have potential to deliver clarithromycin in the gastric medium.116 Kulkarni et al. (2010) developed IPN hydrogel membranes of prazosin hydrochloride using sodium alginate (SA) and PVA as polymers for transdermal delivery. The stiffness of the film was increased by the addition of cross-linker glutaraldehyde and both the stiffness and in vitro drug release property film depend on the concentrations of glutaraldehyde in membranes. The prepared IPN membranes extended prazosin hydrochloride release up to 24h, whereas membranes made up off SA and PVA alone showed fast drug release.117 Microspheres of theophylline were successfully prepared based on IPNs method using chitosan and methylcellulose as polymers and glutaraldehyde as a cross-linker. The encapsulation efficiency was found to be up to 82% and the extended drug release up to 12 h.118 Likewise, duration of 5-fluorouracil release was significantly sustained for up to 52 h from new thermo-sensitive gels in which a chitosan network is crosslinked with various concentrations of glutaraldehyde that interpenetrates PX gels.119
Multicomponent networks as IPNs have better mechanical strength and swelling/deswelling response compared to single-network hydrogels.120 Furthermore, the cross-linking density, hydrogel porosity, and gel stiffness can be adjusted in IPN-based hydrogels according to the target application. The drawbacks are: (i) it has a problem with encapsulating a wide variety of therapeutic agents, especially sensitive bimolecular, and IPN, particularly sequential type, (ii) preparation requires the use of toxic agents such as initiator, activator and cross-linker in order to initiate or catalyze the polymerization or to catalyze the cross-linking.121 Difference between physical and chemical hydrogel and types of cross-linked hydrogel along with their polymer system and drug incorporated is presented in Table 1 and Table 2, respectively.
Table 1. Difference between physical and chemical gel.8 .
| Physical hydrogel | Chemical hydrogel |
| Physical hydrogels are formed by molecular entanglements, and/or secondary forces including ionic, H-bonding or hydrophobic forces. | Chemical hydrogels formed by covalent cross-linking. |
| Above bonds are weak, thus physical hydrogels are considered as reversible gel. | These are termed as permanent or irreversible as covalent bonds are strong. |
| These are prepared without the use of cross-linking entities or chemical modification. | These are prepared using cross-linking entities or chemical modification. |
| It is inflexible towards variables such as gelation time, gel pore size, chemical functionalization, and degradation or dissolution, leading to inconsistent performance in vivo. | It is flexible in respect to gelation time, gel pore size, chemical functionalization, and degradation or dissolution. |
| Physically cross-linked hydrogels are less stable against degradation. | Chemically cross-linked hydrogels are very stable against degradation. |
| These are homogeneous. | These are non-homogeneous. |
| Physical hydrogels have hydrophilic and hydrophobic regions present in the polymeric network. | Chemical hydrogels have domains of high cross-link density as compared to conventional hydrogels. |
| These hydrogels show poor mechanical properties because of the reversible physical interactions. | The mechanical properties of these hydrogels are higher than physically cross-linked hydrogels. |
| For incorporation of bioactive substances, these gels are of great interest. | These are being used in a number of applications like pharmaceutical, agriculture, food industry, cosmetics, etc. |
Table 2. Hydrogel types with their composition and drug incorporated.
| Types of hydrogel | Polymer (s) | Drug | References |
| Hydrophobic interactions | PX 407 | Benzoic acid/ p-hydroxybenzoic acid | 22 |
| PX 188, PX 181 and Pluronic P85 | 2-n-undecylmalonic acid, doxorubicin, ammonium, acetic acid and tetraphenylborate. | 23 | |
| PX 407, Chitosan and Carbopol-934 | Meloxicam | 24 | |
| PX 407 | Indomethacin | 25 | |
| PX 407 | Adriamycin and 5-flurouracil | 26 | |
| PX 407 | Mitomycin C | 27 | |
| HPC and PX 407 | Ketoprofen | 28 | |
| PX 407 and PX 188, Polyvinylmethylether maleic anhydride copolymer, HPMC, PE-400. |
Meloxicam | 30 | |
| HPMC K100M and PX 407 | Procaine | 31 | |
| HPMC and PX 407 | Pranoprofen | 32, 33 | |
| HPMC and PX 407 | Lidocaine HCl | 34 | |
| HPMC and PX 407 | Aceclofenac and Metoprolol succinate | 35, 36 | |
| PNIPAM grafted with hyaluronic acid and chitosan | Riboflavin and 5-fluorouracil | 41, 42 | |
| Chitosan grafted with PEG 40 | BSA | 45 | |
| Alginate solution | BSA | 46 | |
| Charge interactions | HTCC and GP | Doxorubicin HCl | 49 |
| Polyelectrolyte complexes | Chitosan with sodium alginate, carboxymethyl cellulose sodium and k-carrageenan | Metoprolol tartrate | 53 |
| Chitosan and gelatin | ---- | 54 | |
| Chitosan and poly-(γ-glutamic acid) (γ-PGA) | ---- | 55, 56 | |
| Alginate and chitosan | ---- | 57 | |
| Phosphorylated chitosan (a polycation) and chitosan | Osteoblasts | 58 | |
| Polycationic N-trimethyl chitosan and polyanionic N-carboxymethyl chitosan | Dexamethasone | 59 | |
| Quaternized chitosan and PAA | ---- | 60 | |
| Physical mixtures and H-bonding | Carboxymethyl tamarind kernel polysaccharide and PVA | Metronidazole | 64 |
| Chitosan and PVA | Minocycline and Gentamycin | 66, 67 | |
| Microcrystalline cellulose and PVA | Vanillin | 68 | |
| Stereocomplexation | L- and D- PLA | ---- | 70 |
| Isotactic and syndiotactic PMMA | ---- | 71 | |
| D-PLA and L-leuproide | ---- | 72 | |
| Dextran precursors grafted L-lactide and D-lactide oligomers | ---- | 73 | |
| Supramolecular chemistry | PEO polymers and α-cyclodextrins | ---- | 75 |
| β-cyclodextrin & PPO-grafted dextran | ---- | 76 | |
| PEO-poly(R-3-hydroxybutyrate) (PHB)-PEO triblock copolymers & α-cyclodextrin | ---- | 77 | |
| Cross-linking | |||
| (i) Small-molecule cross-linking | Oxidized gum arabic | Primaquine | 79 |
| Genipin | BSA | 86 | |
| Chitosan | Oxprenolol HCl Propanolol HCl 5-Fluorouracil |
87 95 89 |
|
| Chitosan–alginate | Indomethacin | 90 | |
| (ii) Polymer-polymer cross-linking | Hyaluronic acid with amino or aldehyde functionality | Bone morphogenetic protein-2 | 91 |
| Cross-linked hyaluronic acid | Tissue plasminogen activator and budesonide | 93 | |
| Poly(aldehyde guluronate) and adipic acid dihydrazide | Bone precursor cells and growth factor | 94 | |
| (iii) Photo cross-linking | Chitosan-pluronic | Human growth hormone (hGH) Plasmid DNA |
96 97 |
| Modified chitosan-PEG | Growth factors and cells | 98 | |
| (iv) Enzymatic cross-linking | Gelatin cross-linked by mTG | Regenerative cells (HEK293) Interleukin-2 |
100 101 |
| Tyramine modified hyaluronic acid by HRP | Dexamethasone | 103 | |
| Interpenetrating networks | Chitosan, PAA and PVP | Clarithromycin | 116 |
| Chitosan and PVP | Clarithromycin | 117 | |
| SA and PVA | Prazosin HCl | 118 | |
| Chitosan and methylcellulose | Theophylline | 119 | |
| Chitosan and PX | 5-fluorouracil | 120 |
Conclusion and future prospective
Over the past 50 years, there has been continuous progress in designing of hydrogels that led to numerous applications such as pharmaceutical, biomedical, agrochemicals, food industry, etc. In pharmaceutical therapeutic delivery, hydrogels are available in various dosage forms such as tablets, capsules, microspheres, transdermal films, wound dressings, etc. These hydrogels are also used to develop catheter, vascular grafts, semiocclusive dressings, mammary implants, transdermal drug delivery systems, scaffold for tissue engineering and medicated patches etc.6 Despite the wide use, hydrogels suffer significant disadvantages such as low mechanical strength and toughness, difficulties in handling, sterilization, syringability issues. Special structural configurations like slip-link networks, nanocomposite hydrogels, double network hydrogels, multi-functional cross-linked hydrogels, and homogeneous hydrogels can be synthesized to enhance mechanical strength and toughness of resulting hydrogels.122 These techniques should be followed by a suitable method of polymerization or cross-linking in order to make them desirable for suitable application. Another way to enhance mechanical property along with improved biocompatibility and physical property is to combine both physical and chemical cross-linking in a single hydrogel system.
The syringability issue can be solved to a large extent by designing physical gelators which gel at lower polymer concentrations and at more precise gelation temperatures, like in-situ or thermosensitive gels, would reduce the risk of premature gelation inside the needle upon injection. Similarly, for covalently cross-linked hydrogels, the development of strategies to release cross-linker in a triggered manner inside the body, thereby minimizes the risk of syringe clogging, improve the localization of cross-linker release to minimize in vivo toxicity. This strategy is used to transform hydrogels in to “smart” materials that can respond to changes in their environment. In addition the hydrogel properties can be further modified by incorporation of micro or nano fillers. More recently, hydrogels have been modified into hydrogel microparticles for solubility enhancement purposes.123,124
In the present scenario, the focus needs to be shifted towards the development of innovative methods to prepare: (i) hydrophilic polymers of desirable functional groups, (ii) multifunctional/multiarm structures such as grafted or branched co-polymers and star polymers that would offer better properties and suit wider applications in the future.
Acknowledgments
The author thanks management of GITAM University to provide necessary facilities and support.
Ethical Issues
Not applicable.
Conflict of Interest
The author declares no conflicts of interest.
References
- 1.Delgado-Charro MB, Guy RH. Effective use of transdermal drug delivery in children. Adv Drug Deliv Rev. 2014;73:63–82. doi: 10.1016/j.addr.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Raut S, Bhadoriya SS, Uplanchiwar V, Mishra V, Gahane A, Jain SK. Lecithin organogel: A unique micellar system for the delivery of bioactive agents in the treatment of skin aging. Acta Pharm Sin B. 2012;2(1):8–15. doi: 10.1016/j.apsb.2011.12.005. [DOI] [Google Scholar]
- 3. Gunn C. Disperse systems. In: Carter SJ, editor. Tutorial Pharmacy. 6th ed. New Delhi, India: CBS Publishers; 2000. PP. 54-88.
- 4.Vintiloiu A, Leroux JC. Organogels and their use in drug delivery--A review. J Control Release. 2008;125(3):179–92. doi: 10.1016/j.jconrel.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Kumar L, Verma R. In vitro evaluation of topical gel prepared using natural polymer. Int J Drug Deliv. 2010;2(1):58–63. doi: 10.5138/ijdd.2010.0975.0215.02012. [DOI] [Google Scholar]
- 6.Flood P, Page H, Reynaud EG. Using hydrogels in microscopy: A tutorial. Micron. 2016;84:7–16. doi: 10.1016/j.micron.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Singhala R, Gupta K. A Review: Tailor-made hydrogel structures (classifications and synthesis parameters) Polym Plast Technol Eng. 2016;55(1):54–70. doi: 10.1080/03602559.2015.1050520. [DOI] [Google Scholar]
- 8.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54(1):3–12. doi: 10.1016/j.addr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Prestwich GD, Marecak DM, Marecek JF, Vercruysse KP, Ziebell MR. Controlled chemical modification of hyaluronic acid: synthesis, applications, and biodegradation of hydrazide derivatives. J Control Release. 1998;53(1-3):93–103. doi: 10.1016/S0168-3659(97)00242-3. [DOI] [PubMed] [Google Scholar]
- 10.Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater Sci Eng R Rep. 2015;93:1–49. doi: 10.1016/j.mser.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2002;54(1):13–36. doi: 10.1016/S0169-409X(01)00240-X. [DOI] [PubMed] [Google Scholar]
- 12.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–79. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 13.Tessmar JK, Gopferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4-5):274–91. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27–46. doi: 10.1016/S0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2012;64:18–23. doi: 10.1016/j.addr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Berger J, Reist M, Mayer JM, Felt O, Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):35–52. doi: 10.1016/s0939-6411(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 17.Boucard N, Viton C, Domard A. New aspects of the formation of physical hydrogels of chitosan in a hydroalcoholic medium. Biomacromolecules. 2005;6(6):3227–37. doi: 10.1021/bm050653d. [DOI] [PubMed] [Google Scholar]
- 18.Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. doi: 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- 19.Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharm Sci. 2006;9(3):339–58. [PubMed] [Google Scholar]
- 20.Antunes FE, Gentile L, Rossi CO, Tavano L, Ranieri GA. Gels of Pluronic F127 and nonionic surfactants from rheological characterization to controlled drug permeation. Colloids Surf B Biointerfaces. 2011;87(1):42–8. doi: 10.1016/j.colsurfb.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Van Hemelrijck C, Muller-Goymann CC. Rheological characterization and permeation behavior of poloxamer 407-based systems containing 5-aminolevulinic acid for potential application in photodynamic therapy. Int J Pharm. 2012;437(1-2):120–9. doi: 10.1016/j.ijpharm.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert JC, Hadgraft J, Bye A, Brookes LG. Drug release from Pluronic F-127 gels. Int J Pharm. 1986;32(2-3):223–8. doi: 10.1016/0378-5173(86)90182-1. [DOI] [Google Scholar]
- 23.Erukova VY, Krylova OO, Antonenko YN, Melik-Nubarov NS. Effect of ethylene oxide and propylene oxide block copolymers on the permeability of bilayer lipid membranes to small solutes including doxorubicin. Biochim Biophys Acta. 2000;1468(1-2):73–86. doi: 10.1016/S0005-2736(00)00244-3. [DOI] [PubMed] [Google Scholar]
- 24.El-Badry M, Fetih G, Fathalla D, Shakeel F. Transdermal delivery of meloxicam using niosomal hydrogels: in vitro and pharmacodynamic evaluation. Pharm Dev Technol. 2015;20(7):820–6. doi: 10.3109/10837450.2014.926919. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki S, Tobiyama T, Takada M, Attwood D. Percutaneous Absorption of Indomethacin from Pluronic F-127 Gels in Rats. J Pharm Pharmacol. 1995;47:455–7. doi: 10.1111/j.2042-7158.1995.tb05829.x. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki S, Takeuchi S, Yokouchi C, Takada M. Pluronic F-127 gels as a vehicle for Topical administration of Anticancer Agents. Chem Pharm Bull. 1984;32(10):4205–8. doi: 10.1248/cpb.32.4205. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki S, Ohkawa Y, Takada M, Atwood D. Antitumor effect of PF-127 gel containing mytomicin C on sarcoma-180 ascites tumor in mice. Chem Pharm Bull. 1986;34:2224–6. doi: 10.1248/cpb.40.2224. [DOI] [PubMed] [Google Scholar]
- 28.El-Kattan AF, Asbill CS, Kim N, Michniak BB. Effect of formulation variables on the percutaneous permeation of ketoprofen from gel formulations. Drug Deliv. 2000;7(3):147–53. doi: 10.1080/10717540050120188. [DOI] [PubMed] [Google Scholar]
- 29.Chen PC, Kohane DS, Park YJ, Bartlett RH, Langer R, Yang VC. Injectable microparticle-gel system for prolonged and localized lidocaine release. II. In vivo anesthetic effects. J Biomed Mater Res A. 2004;70(3):459–66. doi: 10.1002/jbm.a.30101. [DOI] [PubMed] [Google Scholar]
- 30.Inal O, Yapar EA. Effect of mechanical properties on the release of meloxicam from poloxamer gel bases. Indian J Pharm Sci. 2013;75(6):700–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Jin WG, Shin SC. Preparation and evaluation of procaine gels for the enhanced local anesthetic action. Arch Pharm Res. 2008;31(2):235–41. doi: 10.1007/s12272-001-1147-9. [DOI] [PubMed] [Google Scholar]
- 32.Choi JS, Shin SC. Preparation and evaluation of pranoprofen gel for percutaneous administration. Drug Dev Ind Pharm. 2007;33(1):19–26. doi: 10.1080/03639040600975071. [DOI] [PubMed] [Google Scholar]
- 33.Shin SC, Cho CW. Enhanced transdermal delivery of pranoprofen from the bioadhesive gels. Arch Pharm Res. 2006;29(10):928–33. doi: 10.1007/bf02973916. [DOI] [PubMed] [Google Scholar]
- 34.Shin SC, Cho CW, Yang KH. Development of lidocaine gels for enhanced local anesthetic action. Int J Pharm. 2004;287(1-2):73–8. doi: 10.1016/j.ijpharm.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Parhi R, Garg A. Formulation of topical bioadhesive gel of aceclofenac using 3-level factorial design. Iran J Pharm Res. 2011;10(3):435–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Parhi R. Development and optimization of pluronic® F127 and HPMC based thermosensitive gel for the skin delivery of metoprolol succinate. J Drug Deliv Sci Technol. 2016;36:23–33. doi: 10.1016/j.jddst.2016.09.004. [DOI] [Google Scholar]
- 37.Singh S, Webster DC, Singh J. Thermosensitive polymers: synthesis, characterization, and delivery of proteins. Int J Pharm. 2007;341(1-2):68–77. doi: 10.1016/j.ijpharm.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 38.Lee WC, Li YC, Chu IM. Amphiphilic poly(D,L-lactic acid)/poly(ethylene glycol)/poly(D,L-lactic acid) nanogels for controlled release of hydrophobic drugs. Macromol Biosci. 2006;6(10):846–54. doi: 10.1002/mabi.200600101. [DOI] [PubMed] [Google Scholar]
- 39.Qiao M, Chen D, Ma X, Liu Y. Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm. 2005;294(1-2):103–12. doi: 10.1016/j.ijpharm.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Li C, Tang Y, Armes SP, Morris CJ, Rose SF, Lloyd AW. et al. Synthesis and characterization of biocompatible thermo-responsive gelators based on ABA triblock copolymers. Biomacromolecules. 2005;6(2):994–9. doi: 10.1021/bm049331k. [DOI] [PubMed] [Google Scholar]
- 41.Ha DI, Lee SB, Chong MS, Lee YM, Kim SY, Park YH. Preparation of thermo-responsive and injectable hydrogels based on hyaluronic acid and poly(N-isopropylacrylamide) and their drug release behaviors. Macromol Res. 2006;14(1):87–93. doi: 10.1007/bf03219073. [DOI] [Google Scholar]
- 42.Bae JW, Go DH, Park KD, Lee SJ. Thermosensitive chitosan as an injectable carrier for local drug delivery. Macromol Res. 2006;14(4):461–5. doi: 10.1007/bf03219111. [DOI] [Google Scholar]
- 43.Deng C, Tian H, Zhang P, Sun J, Chen X, Jing X. Synthesis and characterization of RGD peptide grafted poly(ethylene glycol)-b-poly(L-lactide)-b-poly(L-glutamic acid) triblock copolymer. Biomacromolecules. 2006;7(2):590–6. doi: 10.1021/bm050678c. [DOI] [PubMed] [Google Scholar]
- 44.Molinaro G, Leroux JC, Damas J, Adam A. Biocompatibility of thermosensitive chitosan-based hydrogels: an in vivo experimental approach to injectable biomaterials. Biomaterials. 2002;23(13):2717–22. doi: 10.1016/S0142-9612(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 45.Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Control Release. 2005;103(3):609–24. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 46.Liang HF, Hong MH, Ho RM, Chung CK, Lin YH, Chen CH. et al. Novel method using a temperature-sensitive polymer (methylcellulose) to thermally gel aqueous alginate as a pH-sensitive hydrogel. Biomacromolecules. 2004;5(5):1917–25. doi: 10.1021/bm049813w. [DOI] [PubMed] [Google Scholar]
- 47.Uraki Y, Imura T, Kishimoto T, Ubukata M. Body temperature-responsive gels derived from hydroxypropylcellulose bearing lignin. Carbohydr Polym. 2004;58(2):123–30. doi: 10.1016/j.carbpol.2004.05.019. [DOI] [Google Scholar]
- 48.Buwalda SJ, Boere KW, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE. Hydrogels in a historical perspective: From simple networks to smart materials. J Control Release. 2014;190:254–73. doi: 10.1016/j.jconrel.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 49.Wu J, Su ZG, Ma GH. A thermo- and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int J Pharm. 2006;315(1-2):1–11. doi: 10.1016/j.ijpharm.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 50.Van Tomme SR, Van Steenbergen MJ, De Smedt SC, Van Nostrum CF, Hennink WE. Self-gelling hydrogels based on oppositely charged dextran microspheres. Biomaterials. 2005;26(14):2129–35. doi: 10.1016/j.biomaterials.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 51.Chen P. Self-assembly of ionic-complementary peptides: a physicochemical viewpoint. Colloids Surf Physicochem Eng Aspects. 2005;261(1-3):3–24. doi: 10.1016/j.colsurfa.2004.12.048. [DOI] [Google Scholar]
- 52.Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62(1):83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 53.Saleem MA, Kulkarni R, Patil Noornadim G. Formulation and evaluation of chitosan based polyelectrolyte complex hydrogels for extended release of metoprolol tartrate. Res J Pharm Technol. 2011;4(12):1844–51. [Google Scholar]
- 54.Derkach SR, Voronko NG, Sokolan NI. The rheology of hydrogels based on chitosan-gelatin (bio)polyelectrolyte complexes. J Dispersion Sci Technol. 2017;38(10):1427–34. doi: 10.1080/01932691.2016.1250218. [DOI] [Google Scholar]
- 55.Chang HH, Wang YL, Chiang YC, Chen YL, Chuang YH, Tsai SJ. et al. A novel chitosan-γpga polyelectrolyte complex hydrogel promotes early new bone formation in the alveolar socket following tooth extraction. PLoS One. 2014;9(3):e92362. doi: 10.1371/journal.pone.0092362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang HS, Park SH, Lee YG, Son TI. Polyelectrolyte complex hydrogel composed of chitosan and poly(γ-glutamic acid) for biological application: preparation, physical properties, and cytocompatibility. J Appl Polym Sci. 2007;103(1):386–94. doi: 10.1002/app.24623. [DOI] [Google Scholar]
- 57.Kulig D, Zimoch-Korzycka A, Jarmoluk A, Marycz K. Study on alginate-chitosan complex formed with different polymers ratio. Polymers. 2016;8(5):167. doi: 10.3390/polym8050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li QL, Chen ZQ, Darvell BW, Liu LK, Jiang HB, Zen Q. et al. Chitosan-phosphorylated chitosan polyelectrolyte complex hydrogel as an osteoblast carrier. J Biomed Mater Res B Appl Biomater. 2007;82(2):481–6. doi: 10.1002/jbm.b.30753. [DOI] [PubMed] [Google Scholar]
- 59.Zaino C, Zambito Y, Mollica G, Geppi M, Serafini MF, Carelli V. et al. A novel polyelectrolyte complex (PEC) hydrogel for controlled drug delivery to the distal intestine. Open Drug Deliv J. 2007;1:68–75. doi: 10.2174/1874126600701010068. [DOI] [Google Scholar]
- 60.You J, Xie S, Cao J, Ge H, Xu M, Zhang L. et al. Quaternized chitosan/poly(acrylic acid) polyelectrolyte complex hydrogels with tough, self-recovery, and tunable mechanical properties. Macromolecules. 2016;49(3):1049–59. doi: 10.1021/acs.macromol.5b02231. [DOI] [Google Scholar]
- 61.Yao KD, Tu H, Cheng F, Zhang JW, Liu J. pH-sensitivity of the swelling of a chitosan-pectin polyelectrolyte complex. Angew Makromol Chem. 1997;245(1):63–72. doi: 10.1002/apmc.1997.052450106. [DOI] [Google Scholar]
- 62.Ricciardi R, Gaillet C, Ducouret G, Lafuma F, Laupretre F. Investigation of the relationships between the chain organization and rheological properties of atactic poly(vinyl alcohol) hydrogels. Polymer. 2003;44(11):3375–80. doi: 10.1016/S0032-3861(03)00246-5. [DOI] [Google Scholar]
- 63. McGuinness GB, Vrana NE, Liu Y. Polyvinvyl alcohol-based cryogels: tissue engineering and regenerative medicine. In: Mishra M, editor. Encyclopedia of Biomedical Polymers and Polymeric Biomaterials. CRC Press; 2015. PP. 6743-6753.
- 64.Pazos V, Mongrain R, Tardif JC. Polyvinyl alcohol cryogel: Optimizing the parameters of cryogenic treatment using hyperelastic models. J Mech Behav Biomed Mater. 2009;2(5):542–9. doi: 10.1016/j.jmbbm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Meenakshi Meenakshi, Ahuja M. Metronidazole loaded carboxymethyl tamarind kernel polysaccharide-polyvinyl alcohol cryogels: Preparation and characterization. Int J Biol Macromol. 2015;72:931–8. doi: 10.1016/j.ijbiomac.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 66.Sung JH, Hwang MR, Kim JO, Lee JH, Kim YI, Kim JH. et al. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int J Pharm. 2010;392(1-2):232–40. doi: 10.1016/j.ijpharm.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 67.Hwang MR, Kim JO, Lee JH, Kim YI, Kim JH, Chang SW. et al. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. AAPS PharmSciTech. 2010;11(3):1092–103. doi: 10.1208/s12249-010-9474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paduraru OM, Ciolacu D, Darie RN, Vasile C. Synthesis and characterization of polyvinyl alcohol/cellulose cryogels and their testing as carriers for a bioactive component. Mater Sci Eng C. 2012;32(8):2508–15. doi: 10.1016/j.msec.2012.07.033. [DOI] [Google Scholar]
- 69.Lapasin R, Pricl S. Rheology of industrial polysaccharides: theory and application. Cornwall, UK: Blackie Academic and Professional; 1995. [Google Scholar]
- 70.Tsuji H. Poly(lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications. Macromol Biosci. 2005;5(7):569–97. doi: 10.1002/mabi.200500062. [DOI] [PubMed] [Google Scholar]
- 71.Fox TG, Garrett BS, Goode WE, Gratch S, Kincaid JF, Spell A. et al. Crystalline polymers of methyl methacrylate. J Am Chem Soc. 1958;80(7):1768–9. doi: 10.1021/ja01540a068. [DOI] [Google Scholar]
- 72.Slager J, Glandnikoff M, Domb AJ. Stereocomplexes, based on biodegradable polymers and bioactive macromolecules. Macromol Symp. 2001;175(1):105–15. [Google Scholar]
- 73.Bos GW, Jacobs JJ, Koten JW, Van Tomme S, Veldhuis T, van Nostrum CF. et al. In situ crosslinked biodegradable hydrogels loaded with IL-2 are effective tools for local IL-2 therapy. Eur J Pharm Sci. 2004;21(4):561–7. doi: 10.1016/j.ejps.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Oshovsky GV, Reinhoudt DN, Verboom W. Supramolecular Chemistry in Water. Angew Chem Int Ed Engl. 2007;46(14):2366–93. doi: 10.1002/anie.200602815. [DOI] [PubMed] [Google Scholar]
- 75.Huh KM, Ooya T, Lee WK, Sasaki S, Kwon IC, Jeong SY. et al. Supramolecular-structured hydrogels showing a reversible phase transition by inclusion complexation between poly(ethylene glycol) grafted dextran and α-cyclodextrin. Macromolecules. 2001;34(25):8657–62. doi: 10.1021/ma0106649. [DOI] [Google Scholar]
- 76.Choi HS, Kontani K, Huh KM, Sasaki S, Ooya T, Lee WK. et al. Rapid induction of thermoreversible hydrogel formation based on poly(propylene glycol)-grafted dextran inclusion complexes. Macromol Biosci. 2002;2(6):298–303. [Google Scholar]
- 77.Li J, Li X, Ni X, Wang X, Li H, Leong KW. Self-assembled supramolecular hydrogels formed by biodegradable PEO-PHB-PEO triblock copolymers and alpha-cyclodextrin for controlled drug delivery. Biomaterials. 2006;27(22):4132–40. doi: 10.1016/j.biomaterials.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 78.Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):19–34. doi: 10.1016/S0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 79.Nishi KK, Jayakrishnan A. Self-gelling primaquine-gum arabic conjugate: an injectable controlled delivery system for primaquine. Biomacromolecules. 2007;8(1):84–90. doi: 10.1021/bm060612x. [DOI] [PubMed] [Google Scholar]
- 80.Shim WS, Kim JH, Kim K, Kim YS, Park RW, Kim IS. et al. pH- and temperature-sensitive, injectable, biodegradable block copolymer hydrogels as carriers for paclitaxel. Int J Pharm. 2007;331(1):11–8. doi: 10.1016/j.ijpharm.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 81.Jin R, Hiemstra C, Zhong Z, Feijen J. Enzyme-mediated fast in situ formation of hydrogels from dextran-tyramine conjugates. Biomaterials. 2007;28(18):2791–800. doi: 10.1016/j.biomaterials.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 82.Kurisawa M, Chung JE, Yang YY, Gao SJ, Uyama H. Injectable biodegradable hydrogels composed of hyaluronic acid-tyramine conjugates for drug delivery and tissue engineering. Chem Commun. 2005;(34):4312–4. doi: 10.1039/b506989k. [DOI] [PubMed] [Google Scholar]
- 83.Jin J, Song M, Hourston DJ. Novel chitosan-based films cross-linked by genipin with improved physical properties. Biomacromolecules. 2004;5(1):162–8. doi: 10.1021/bm034286m. [DOI] [PubMed] [Google Scholar]
- 84.Sung HW, Liang IL, Chen CN, Huang RN, Liang HF. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin) J Biomed Mater Res. 2001;55(4):538–46. doi: 10.1002/1097-4636(20010615)55:4<538::aid-jbm1047>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 85.Chen SC, Wu YC, Mi FL, Lin YH, Yu LC, Sung HW. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J Control Release. 2004;96(2):285–300. doi: 10.1016/j.jconrel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 86.Butler MF, Ng YF, Pudney PDA. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J Polym Sci A Polym Chem. 2003;41(24):3941–53. doi: 10.1002/pola.10960. [DOI] [Google Scholar]
- 87.Bolgul Y, Hekimoglu S, Sahinerdemli I, Kas HS. Evaluation of oxprenolol hydrochloride permeation through isolated human skin and pharmacodynamic effect in rats. STP Pharm Sci. 1998;8(3):197–201. [Google Scholar]
- 88.Thacharodi D, Rao KP. Development and in vitro evaluation of chitosan-based transdermal drug delivery systems for the controlled delivery of propranolol hydrochloride. Biomaterials. 1995;16(2):145–8. doi: 10.1016/0142-9612(95)98278-M. [DOI] [PubMed] [Google Scholar]
- 89.Denkbas EB, Seyyal M, Piskin E. Implantable 5-fluorouracil loaded chitosan scaffolds prepared by wet spinning. J Memb Sci. 2000;172(1-2):33–8. doi: 10.1016/S0376-7388(00)00314-8. [DOI] [Google Scholar]
- 90.Mi FL, Sung HW, Shyu SS. Drug release from chitosan-alginate complex beads reinforced by a naturally occurring cross-linking agent. Carbohydr Polym. 2002;48(1):61–72. doi: 10.1016/S0144-8617(01)00212-0. [DOI] [Google Scholar]
- 91.Hiemstra C, van der Aa LJ, Zhong ZY, Dijkstra PJ, Feijen J. Novel in situ forming, degradable dextran hydrogels by michael addition chemistry: Synthesis, rheology, and degradation. Macromolecules. 2007;40(4):1165–73. doi: 10.1021/ma062468d. [DOI] [Google Scholar]
- 92.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47(2):152–69. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 93.Ito T, Yeo Y, Highley CB, Bellas E, Benitez CA, Kohane DS. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials. 2007;28(6):975–83. doi: 10.1016/j.biomaterials.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee KY, Alsberg E, Mooney DJ. Degradable and injectable poly(aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res. 2001;56(2):228–33. doi: 10.1002/1097-4636(200108)56:2<228::aid-jbm1089>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 95.Ono K, Saito Y, Yura H, Ishikawa K, Kurita A, Akaike T. et al. Photocrosslinkable chitosan as a biological adhesive. J Biomed Mater Res. 2000;49(2):289–95. doi: 10.1002/(sici)1097-4636(200002)49:2<289::aid-jbm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 96.Yoo HS. Photo-cross-linkable and thermo-responsive hydrogels containing chitosan and pluronic for sustained release of human growth hormone (hGH) J Biomater Sci Polym Ed. 2007;18(11):1429–41. doi: 10.1163/156856207782246803. [DOI] [PubMed] [Google Scholar]
- 97.Lee JI, Kim HS, Yoo HS. DNA nanogels composed of chitosan and Pluronic with thermo-sensitive and photo-crosslinking properties. Int J Pharm. 2009;373(1-2):93–9. doi: 10.1016/j.ijpharm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 98.Yeo Y, Geng W, Ito T, Kohane DS, Burdick JA, Radisic M. Photocrosslinkable hydrogel for myocyte cell culture and injection. J Biomed Mater Res B Appl Biomater. 2007;81(2):312–22. doi: 10.1002/jbm.b.30667. [DOI] [PubMed] [Google Scholar]
- 99.Lukaszczyk J, Smiga M, Jaszcz K, Adler HJ, Jahne E, Kaczmarek M. Evaluation of oligo(ethylene glycol) dimethacrylates effects on the properties of new biodegradable bone cement compositions. Macromol Biosci. 2005;5(1):64–9. doi: 10.1002/mabi.200400135. [DOI] [PubMed] [Google Scholar]
- 100.Yung CW, Wu LQ, Tullman JA, Payne GF, Bentley WE, Barbari TA. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J Biomed Mater Res A. 2007;83(4):1039–46. doi: 10.1002/jbm.a.31431. [DOI] [PubMed] [Google Scholar]
- 101.Yung CW, Bentley WE, Barbari TA. Diffusion of interleukin-2 from cells overlaid with cytocompatible enzyme-crosslinked gelatin hydrogels. J Biomed Mater Res A. 2010;95(1):25–32. doi: 10.1002/jbm.a.32740. [DOI] [PubMed] [Google Scholar]
- 102.Davis NE, Ding S, Forster RE, Pinkas DM, Barron AE. Modular enzymatically crosslinked protein polymer hydrogels for in situ gelation. Biomaterials. 2010;31(28):7288–97. doi: 10.1016/j.biomaterials.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim KS, Park SJ, Yang JA, Jeon JH, Bhang SH, Kim BS. et al. Injectable hyaluronic acid-tyramine hydrogels for the treatment of rheumatoid arthritis. Acta Biomater. 2011;7(2):666–74. doi: 10.1016/j.actbio.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 104.Jin R, Moreira Teixeira LS, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY. et al. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2544–51. doi: 10.1016/j.biomaterials.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 105.Chen T, Embree HD, Brown EM, Taylor MM, Payne GF. Enzyme-catalyzed gel formation of gelatin and chitosan: potential for in situ applications. Biomaterials. 2003;24(17):2831–41. doi: 10.1016/S0142-9612(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 106.Teixeira LS, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials. 2012;33(5):1281–90. doi: 10.1016/j.biomaterials.2011.10.067. [DOI] [PubMed] [Google Scholar]
- 107.Chen T, Embree HD, Wu LQ, Payne GF. In vitro protein-polysaccharide conjugation: tyrosinase-catalyzed conjugation of gelatin and chitosan. Biopolymers. 2002;64(6):292–302. doi: 10.1002/bip.10196. [DOI] [PubMed] [Google Scholar]
- 108.Singh P, Kumar SK, Keerthi TS, Mani TT, Getyala A. Interpenetrating polymer network (IPN) microparticles and advancement in novel drug delivery system: a review. Pharm Sci Monitor. 2012;3(1):1826–37. [Google Scholar]
- 109.Rokhade AP, Patil SA, Aminabhavi TM. Synthesis and characterization of semi-interpenetrating polymer network microspheres of acrylamide grafted dextran and chitosan for controlled release of acyclovir. Carbohydr Polym. 2007;67(4):605–13. doi: 10.1016/j.carbpol.2006.07.001. [DOI] [Google Scholar]
- 110.Myung D, Waters D, Wiseman M, Duhamel PE, Noolandi J, Ta CN. et al. Progress in the development of interpenetrating polymer network hydrogels. Polym Adv Technol. 2008;19(6):647–57. doi: 10.1002/pat.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Margaret MT, Brahmaiah B, Krishna PV, Revathi B, Nama S. Interpenetrating polymer network (IPN) microparticles an advancement in novel drug delivery system: a review. Int J Pharm Res Bio Sci. 2013;2(3):215–24. [Google Scholar]
- 112. Kudela V. Hydrogels. In: Kroschwitz JI, editor. Encyclopedia of polymer science and engineering. New York: Wiley; 1987. PP. 783-807.
- 113.Qadri MF, Malviya R, Sharma PK. Biomedical applications of interpenetrating polymer network system. Open Pharm Sci J. 2015;2:21–30. doi: 10.2174/1874844901502010021. [DOI] [Google Scholar]
- 114.Dragan ES. Design and applications of interpenetrating polymer network hydrogels. A review. Chem Eng J. 2014;243:572–90. doi: 10.1016/j.cej.2014.01.065. [DOI] [Google Scholar]
- 115.Kumar GA, Wadood SA, Datta MS, Ramchand D. Interpenetrating polymeric network hydrogel for stomach-specific drug delivery of clarithromycin: Preparation and evaluation. Asian J Pharm. 2010;4(4):179–84. doi: 10.4103/0973-8398.76738. [DOI] [Google Scholar]
- 116.Vaghani SS, Patel MM. pH-sensitive hydrogels based on semi-interpenetrating network (semi-IPN) of chitosan and polyvinyl pyrrolidone for clarithromycin release. Drug Dev Ind Pharm. 2011;37(10):1160–9. doi: 10.3109/03639045.2011.563422. [DOI] [PubMed] [Google Scholar]
- 117.Kulkarni RV, Sreedhar V, Mutalik S, Setty CM, Sa B. Interpenetrating network hydrogel membranes of sodium alginate and poly(vinyl alcohol) for controlled release of prazosin hydrochloride through skin. Int J Biol Macromol. 2010;47(4):520–7. doi: 10.1016/j.ijbiomac.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 118.Rokhade AP, Shelke NB, Patil SA, Aminabhavi TM. Novel interpenetrating polymer network microspheres of chitosan and methylcellulose for controlled release of theophylline. Carbohydr Polym. 2007;69(4):678–87. doi: 10.1016/j.carbpol.2007.02.008. [DOI] [Google Scholar]
- 119.Chung TW, Lin SY, Liu DZ, Tyan YC, Yang JS. Sustained release of 5-FU from Poloxamer gels interpenetrated by crosslinking chitosan network. Int J Pharm. 2009;382(1-2):39–44. doi: 10.1016/j.ijpharm.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, Liu J, Huang L, Wang Z, Wang L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci Rep. 2015;5:12374. doi: 10.1038/srep12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res. 2015;6(2):105–21. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Naficy S, Kawakami S, Sadegholvaad S, Wakisaka M, Spinks GM. Mechanical properties of interpenetrating polymer network hydrogels based on hybrid ionically and covalently crosslinked networks. J Appl Polym Sci. 2013;130(4):2504–13. doi: 10.1002/app.39417. [DOI] [Google Scholar]
- 123.Mahmood A, Ahmad M, Sarfraz RM, Minhas MU. β-CD based hydrogel microparticulate system to improve the solubility of acyclovir: Optimization through in-vitro, in-vivo and toxicological evaluation. J Drug Deliv Sci Technol. 2016;36:75–88. doi: 10.1016/j.jddst.2016.09.005. [DOI] [Google Scholar]
- 124. Mahmood A, Ahmad M, Sarfraz RM, Minhas MU. Development of Acyclovir Loaded β-Cyclodextrin-g-Poly Methacrylic Acid Hydrogel Microparticles: An In Vitro Characterization. Adv Polym Technol 2016:21711.


