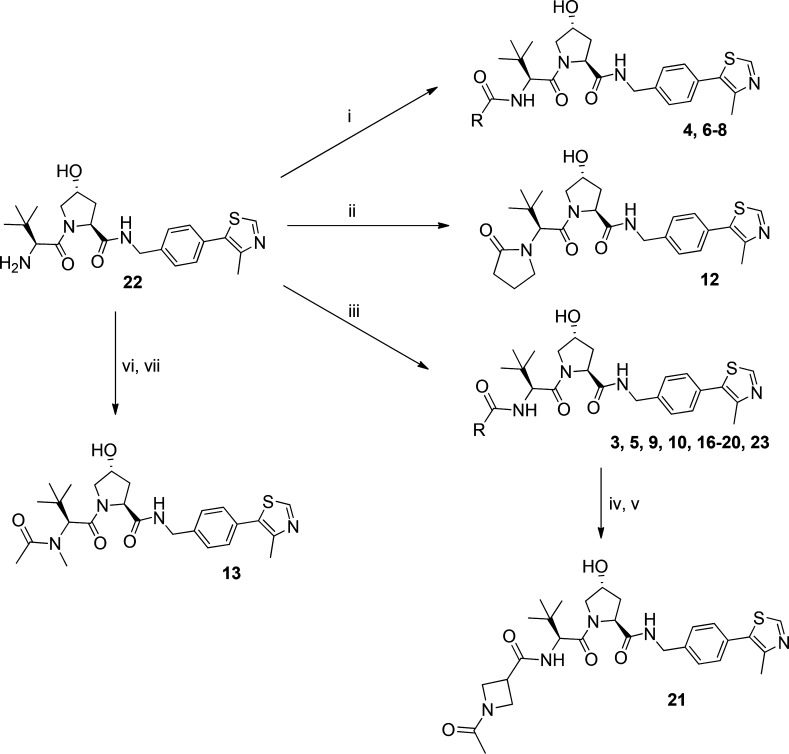
Scheme 1.
Reagents and conditions: (i) N(Et)3, anhydride derivative, DCM, rt, 2 h; (ii) 4-chlorobutanoyl chloride, DCM/NaOH, rt, 1 h; KOtBu, THF, 0 °C–rt, on; (iii) carboxylic acid derivative, HATU, DIPEA, DMF, rt, 1 h; (iv) TFA:DCM, rt, 30 min; (v) N(Et)3, acetic anhydride, DCM, rt, 2 h; (vi) formaldehyde, DMF, rt, 90 min, NaBH(OAc)3, rt, 10 min; (vii) N(Et)3, acetic anhydride, DCM, rt, 2 h.