Figure 7.
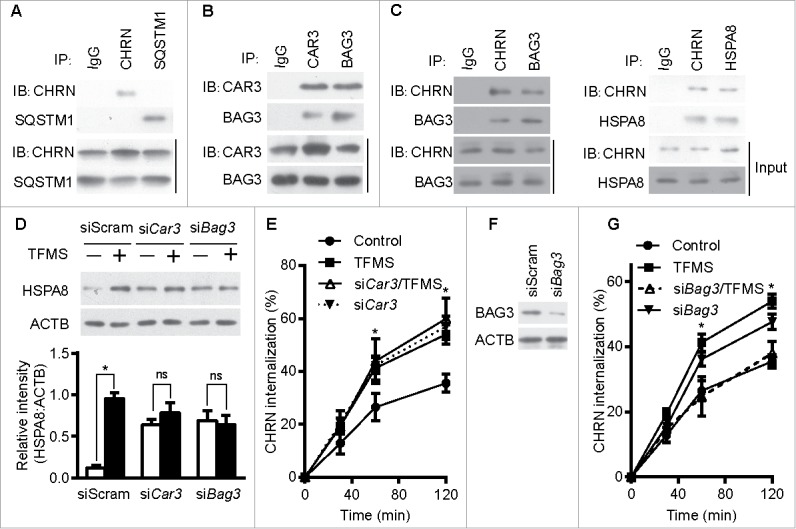
CAR3 suppresses endocytosis by repressing chaperone-assisted selective autophagy. (A) C2C12 cells were lysed, immunoprecipitated with the indicated antibody, then blotted with the specified antibodies. (B and C) C2C12 cells were lysed, followed by immunoprecipitation with the indicated antibody, and then blotted with the specified antibodies. (D) C2C12 cells were transiently transfected with specific siRNA using Lipofectamine 3000. Forty-eight h later, the cells were treated with TFMS (2 mM) for 6 h. Cell lysates from these cells were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. (E) C2C12 cells were transiently transfected with specific siRNA against Car3 using Lipofectamine 3000. Forty-eight h later, the cells with treated with TFMS (2 mM) for 6 h followed by incubation with CHRN antibody (mAb210) at 4°C for 1 h, and then switched to 37°C for different times to induce CHRN endocytosis. After acidic washes, the cells were fixed and analyzed with flow cytometry. (F) C2C12 cells were transiently transfected with siScram or siBag3 using Lipofectamine 3000. Forty-eight h later, the cells were lysed and subjected to SDS-PAGE and analyzed by immunoblotting with anti-BAG3 antibody. (G) C2C12 cells were transiently transfected with specific siRNA (siBag3) using Lipofectamine 3000. Forty-eight h later, either control C2C12 cells or siBag3-transfected cells were treated with vehicle or TFMS (2 mM) for 6 h followed by incubation with CHRN antibody (mAb210) at 4°C for 1 h, and then switched to 37°C for different times to induce CHRN endocytosis. After acidic washes, the cells were fixed and analyzed with flow cytometry. All immunoblotting and immunoprecipitation studies were performed 3 times. *p < 0.05, compared with the control group. Data are mean ± SEM of 3 independent experiments.
