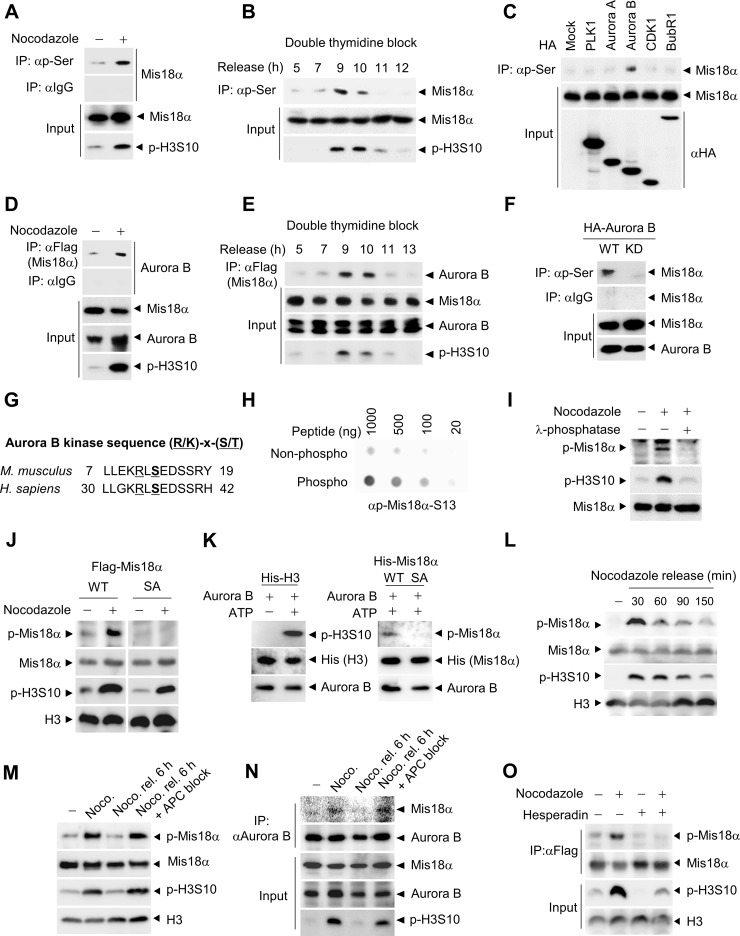
Figure 1. Mis18α is phosphorylated during mitosis by Aurora B kinase.
(A) HeLa cells stably expressing Flag-Mis18α (HeLa/Flag-Mis18α) were synchronized by nocodazole treatment. Cell extracts were subjected to immunoprecipitation (IP) with an antibody against phosphorylated-serine (p-Ser) followed by immunoblotting with anti-Flag antibody. Phosphorylation of 10th serine residue of histone H3 (p-H3S10) was used as a mitosis indicator. (B) HeLa/Flag-Mis18α cells were synchronized at G1/S by double thymidine block and released into indicated time points and were analyzed as in (A). (C) Mitotic kinases were transfected into HeLa/Flag-Mis18α cells and cell extracts were applied to IP with the anti-p-Ser antibody followed by immunoblotting with anti-Flag antibody. (D) Mitotically arrested HeLa/Flag-Mis18α cells with nocodazole treatment were applied for IP with anti-Flag antibody and detected with anti-Aurora B antibody. (E) HeLa/Flag-Mis18α cells prepared as in B were used for IP assay with anti-Flag antibody and detected with anti-Aurora B antibody. (F) HeLa/Flag-Mis18α cells transfected with Aurora B wild-type (Aurora B WT) or K160A kinase dead mutant (Aurora B KD) were used for IP with anti-p-Ser antibody. (G) Aurora B kinase consensus sequences in mouse and human Mis18α. (H) Dot blot analysis for a phosphorylation-specific antibody of Mis18α on Ser36 (p-Mis18α) by comparing non-phospho peptide with phospho-peptide at indicated concentrations. (I) Extracts from 293T cells transfected with Flag-Mis18α were treated with λ-phosphatase and used for immunoblotting with anti-p-Mis18α antibody. (J) 293T cells were transfected with Flag-Mis18α WT, Flag-Mis18αSA and synchronized by nocodazole treatment. Cell extracts were used for immunoblotting with anti-p-Mis18α antibody. (K) Recombinant His-H3 or His-Mis18α were incubated with purified Aurora B kinase in the presence of ATP for 30 min at 30°C for in vitro kinase assay. Phosphorylation of Mis18α was detected using anti-p-Mis18α. (L) 293T cells expressing Flag-Mis18α WT were synchronized by nocodazole treatment. After releasing, cells were harvested at indicated time points and applied for immunoblotting. (M) 293T cells expressing Flag-Mis18α WT were released for 6 h from nocodazole-mediated synchronization with or without MG132 treatment to block APC activity. (N) HeLa/Flag-Mis18α cells were released for 6 h from nocodazole-mediated synchronization as in M and subject to IP analysis with anti-Aurora B antibody. (O) HeLa/Flag-Mis18α cells expressing Flag-Mis18α were treated with Aurora B kinase inhibitor, Hesperadin and the phosphorylation of Mis18α was evaluated by using anti-p-Mis18α antibody under nocodazole treatment.