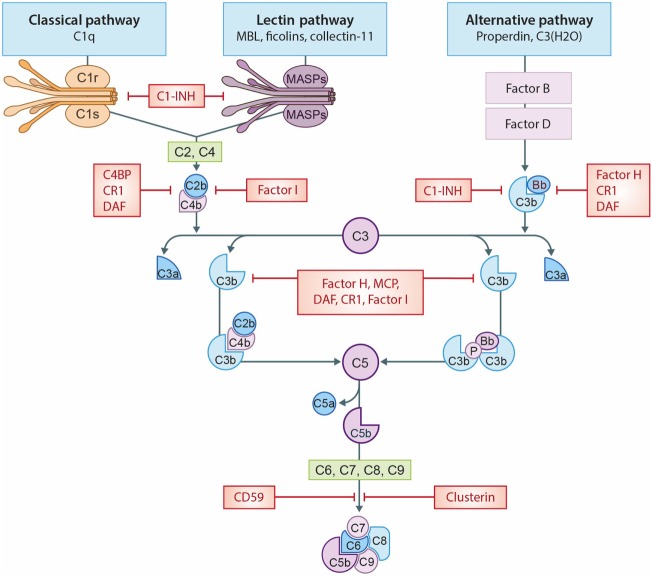
Figure 1.
The complement system. A schematic view of activation of the complement system and its regulation. The classical pathway (CP) is initiated by C1q binding to immune complexes or other molecules (e.g., CRP), thereby activating C1r and C1s resulting in the cleavage of C2 and C4 thereby forming the C3-convertase (C4b2b). The lectin pathway (LP) is initiated by mannose-binding lectin (MBL), ficolins, or collectin-11 binding to carbohydrates or other molecules (e.g., IgA), thereby activating MASP-1 and MASP-2, forming the same C3-convertase as the CP. Subsequently, the C3-convertase cleavages C3 into C3a and C3b. Activation of the alternative pathway (AP) occurs via properdin binding to certain cell surfaces (e.g., LPS) or by spontaneous hydrolysis of C3 into C3(H2O). Next, binding of factor B creates the AP C3-convertase (C3bBb). Increased levels of C3b results in the formation of the C5-convertases, which cleaves C5 in C5a, a powerful anaphylatoxin, and C5b. Next, C5b binds to the surface and interactions with C6–C9, generating the membrane attack complexes (MAC/C5b-9). Several complement regulators (either soluble and membrane-bound) prevent or restrain complement activation. C1 esterase inhibitor (C1-INH) inhibits the activation of early pathway activation of all three pathways, while C4b-binding protein (C4BP) controls activation at the C4 level of the CP and LP. Factor I and factor H regulate the C3 and C5-convertase. Furthermore, the membrane-bound inhibitors include complement receptor 1 (CR1), membrane cofactor protein (MCP) that acts as an co-factors for factor I and decay accelerating factor (DAF) which accelerates the decay of C3-convertases. The membrane-bound regulator Clusterin and CD59 prevents the generation of the C5b-9.