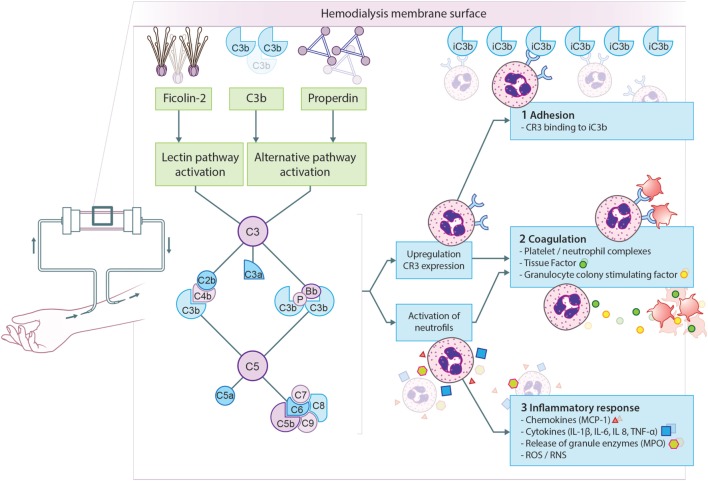
Figure 2.
Proposed model for complement activation in hemodialysis (HD). The principal mechanism leading to complement activation in HD is the binding of ficolin-2 to the membrane, resulting in lectin pathway activation. Simultaneously, properdin and/or C3b bind to the membrane resulting in alternative pathway activation. Complement activation will result in the formation of anaphylatoxins (C3a, C5a), opsonins (C3b, iC3b), and the membrane attack complex (C5b-9). First, complement activation leads to the upregulation of complement receptor 3 (CR3) allowing leukocytes to bind C3 fragments deposited on the membrane, leading to leukopenia. Second, CR3 on neutrophils is also important for the formation of platelet-neutrophil complexes, which contributes to thrombotic processes. Furthermore, C5a generation during HD leads to the expression of tissue factor and granulocyte colony-stimulating factor in neutrophils, shifting HD patients to a procoagulant state. Third, complement activation also promotes recruitment and activation of leukocytes resulting in the oxidative burst and the release of pro-inflammatory cytokines and chemokine’s. More specifically, the activation of neutrophils by C5a leads to the release of granule enzymes, e.g., myeloperoxidase (MPO).