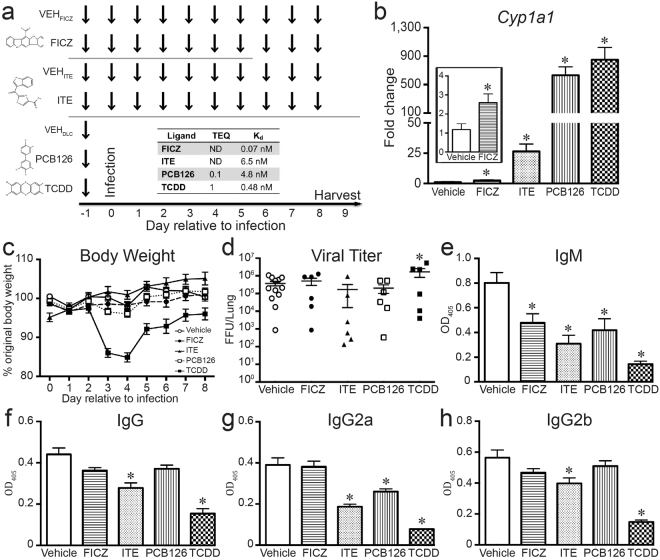
Figure 1.
In vivo administration activates AHR. (a) Dosing strategy: arrows depict when female C57Bl/6 mice were treated with each compound. The indicated times are relative to intranasal (i.n.) infection with IAV, which is denoted as day 0. TCDD (10 μg/kg BW) and PCB126 (100 μg/kg BW) were administered orally once, one day before infection. FICZ (100 μg/kg BW daily) was also administered by gavage, whereas ITE (10 mg/kg BW daily) was given intraperitoneally (i.p.). Structures for each compound are shown to the left of the dosing strategy (www.chemspider.com). Control mice received the appropriate vehicle following the same treatment route and dosing schedule: VEHFICZ, VEHITE, VEHDLC. The response of all vehicle treatment groups to infection was not different; therefore, a single representative vehicle group is shown in all figures. (b) RNA was isolated and RT-qPCR was performed to measure Cyp1a1 levels. The graph depicts the mean expression of Cyp1a1 levels in liver. The inset graph shows an enlargement of data comparing FICZ and vehicle treated mice. (c) The graph depicts the percent body weight change relative to the day prior to infection for mice in all treatment groups. (d) The pulmonary viral burden was measured 2 days after infection. The viral FFU per lung was determined by incubating lung homogenates on MDCK cells. Each symbol represents FFU/lung from a different mouse, and the horizontal line denotes the mean FFU for each treatment group. (e–h) Anti-influenza virus antibody ELISAs were performed using a dilution series of serum. The graphs show the mean level of circulating virus-specific (e) IgM (f) IgG, (g) IgG2a and (h) IgG2b in each group at the same serum dilution (1:6400). 5–8 mice were used per treatment group, and an * indicates a p value ≤ 0.05 as compared to appropriate vehicle control. All data are presented as the mean ± SEM. All experiments have been independently repeated at least once with similar results.