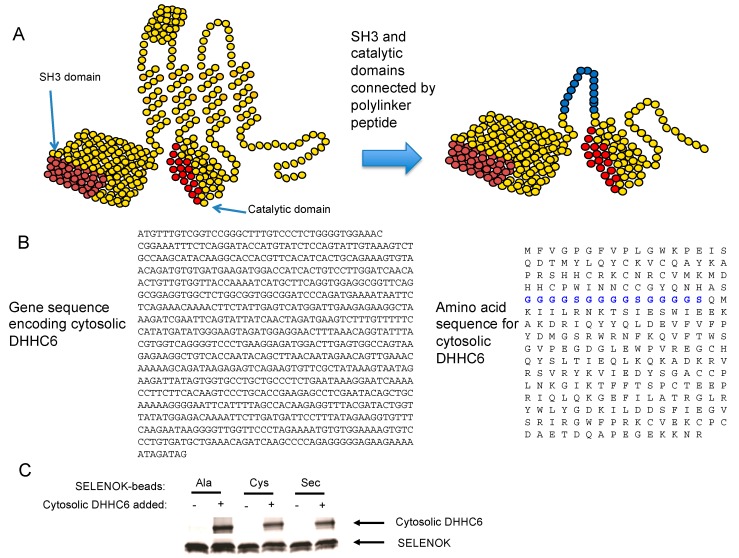
Figure 3.
Synthesis of a soluble cytosolic DHHC6 that binds to SELENOK. (A) Illustration of full-length DHHC6 with the SH3 and catalytic domains that were used to construct a modified version of DHHC6, which contains both domains connected by a linker domain shown in blue. (B) The DNA sequence used to express the cytosolic DHHC6 along with amino acid sequence showing linker region in blue. (C) StrepTactin beads were used to pull down 1 μg streptavidin-tagged SELENOK containing either Ala, or Cys, or Sec at amino acid position 92. These beads were divided into two aliquots that were not or were incubated overnight at 4 °C with the cytosolic DHHC6 in a 1:1 molar ratio. Eluted proteins were analyzed by Coomassie blue staining of PAGE, and results show all three versions of SELENOK bound to cytosolic DHHC6. Note that some degradation of the recombinant SELENOK occurred during the overnight incubation giving rise to double bands. Also, these are recombinant proteins and not cell lysates, which leads to no detectable background in lanes corresponding to the “no Cytosolic DHHC6 added” conditions.