Figure 2.
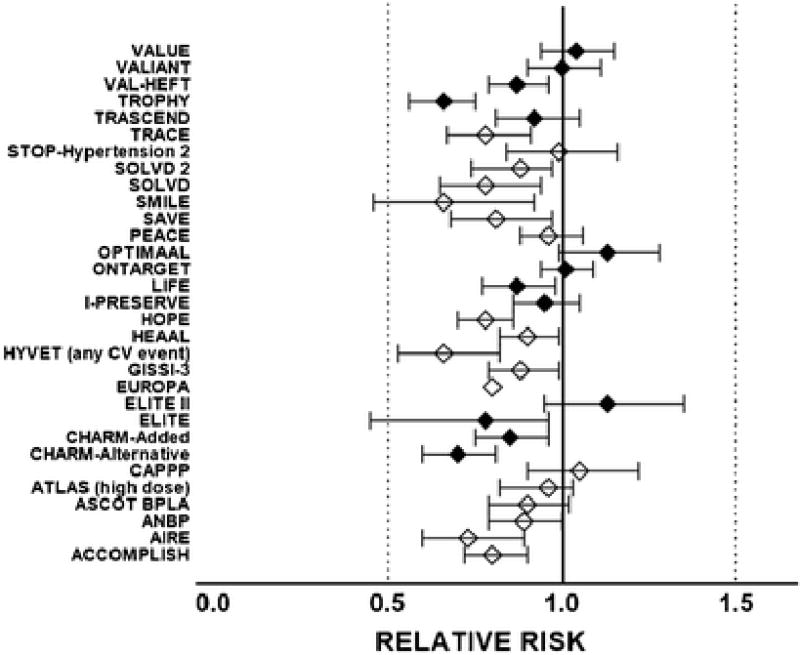
Relative risk and 95 % confidence intervals of the effect of angiotensin converting enzyme inhibitors (open diamonds) or Ang II receptor blockers (closed diamonds) on primary cardiac end points of large randomized clinical trials. Overall, the reduction in the primary end-point across all the trials documented here averaged 0.87 (CI, 0.83 – 0.92). Acronyms are: ACCOMPLISH, Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension [96]; AIRE, Acute Infarction Ramipril Efficacy [97]; ANBP-2, Second Australian National Blood Pressure Study Group [98]; ASCOT BPLA, Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm [99]; ATLAS (high dose), Assessment of Treatment with Lisinopril And Survival [100]; CAPPP, Captopril Prevention Project [101]; CHARM-Alternative, Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity [77]; CHARM-Added, Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity [79]; ELITE, Evaluation of Losartan in the Elderly Study [102]; ELITE II, the Losartan Heart Failure Survival Study (Evaluation of Losartan in the Elderly Study) [103]; EUROPA, European trial on Reduction Of cardiac events with Perindopril in patients with stable coronary Artery disease [104]; GISSI-3, Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico [105]; HYVET, Hypertension in the Very Elderly Trial [106]; HEAAL, Heart failure Endpoint evaluation of Ang II Antagonist Losartan [107]; HOPE, Heart Outcomes Prevention Evaluation Study [108]; I-PRESERVE, Irbesartan in Heart Failure with Preserved Ejection Fraction Study [109]; LIFE, Losartan Intervention For Endpoint reduction Study [78]; ONTARGET, The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial [110]; OPTIMAAL, Optimal Trial in Myocardial Infarction with the Ang II Antagonist Losartan [111]; PEACE, Prevention of Events with Angiotensin Converting Enzyme Inhibition [112]; SAVE, Survival and Ventricular Enlargement trial [113]; SMILE, Survival of Myocardial Infarction Long-Term Evaluation trial [114]; SOLVD, Studies of Left Ventricular Dysfunction [76]; SOLVD 2, Studies of Left Ventricular Dysfunction [115]; STOP-Hypertension 2, Swedish Trial in Old Patients with Hypertension-2 study [116]; TRACE, Trandolapril Cardiac Evaluation [117]; TRASCEND, Telmisartan Randomised Assessmen Study in ACE Intolerant subjects with cardiovascular Disease [118]; TROPHY, Trial Preventing Hypertension [119]; VAL-HEFT, Valsartan Heart Failure Trial [120]; VALIANT, Valsartan in Acute Myocardial Infarction trial [121]; VALUE, Valsartan Antihypertensive Long-term Use Evaluation study [122].
