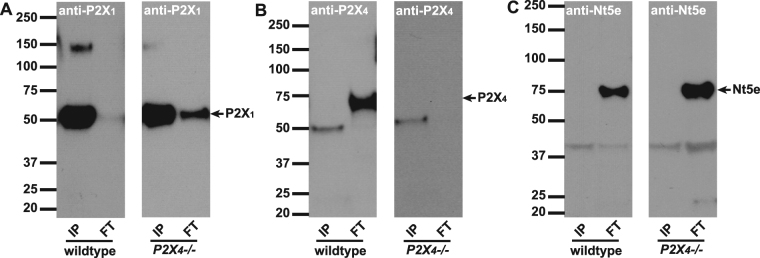
Figure 2.
Immunoprecipitation with anti-P2X1 antibody. Antibodies to P2X1 were immobilized onto resin beads and then incubated with mouse bladder lysates to IP the antigen and co-IP interacting proteins. Proteins that were bound (IP: 2.5 µg protein/lane) or did not bind (FT: 25 µg protein/lane) to the beads were resolved by SDS-PAGE, and Western blots were probed with A) P2X1, B) P2X4 or C) Nt5e antibodies. (A) Left and right panels show P2X1 immunoblots on IP and FT lysates from wild type and P2X4−/− mice. Monomeric P2X1 can be seen highly concentrated in the pulldown fraction at 50 kDa. Little P2X1 appears in FT. (B) P2X4 antibody detects P2X4 as a band at 70 kDa in wild type, but is completely absent in P2X4−/− mice. The antibody shows minor cross-reactivity to possibly P2X1. Note, there is no evidence of the 70 kDa band in the IP lane. (C) An antibody to 5′-nucleotidase (Nt5e) demonstrates that pulldown with anti-P2X1 is ‘clean’ with no non-specific protein binding evident in the IP lanes.