Abstract
Genetic diversity confers adaptive capacity to populations under changing conditions but its role in mediating impacts of climate change remains unresolved for most ecosystems. This lack of knowledge is particularly acute for foundation species, where impacts may cascade throughout entire ecosystems. We combined population genetics with eco-physiological and ecological field experiments to explore relationships among latitudinal patterns in genetic diversity, physiology and resilience of a kelp ecosystem to climate stress. A subsequent ‘natural experiment’ illustrated the possible influence of latitudinal patterns of genetic diversity on ecosystem vulnerability to an extreme climatic perturbation (marine heatwave). There were strong relationships between physiological versatility, ecological resilience and genetic diversity of kelp forests across latitudes, and genetic diversity consistently outperformed other explanatory variables in contributing to the response of kelp forests to the marine heatwave. Population performance and vulnerability to a severe climatic event were thus strongly related to latitudinal patterns in genetic diversity, with the heatwave extirpating forests with low genetic diversity. Where foundation species control ecological structure and function, impacts of climatic stress can cascade through the ecosystem and, consequently, genetic diversity could contribute to ecosystem vulnerability to climate change.
Introduction
A core tenet of evolutionary theory is that the ability of species to adapt and persist through changing environments is contingent on latent functional responses suited to new conditions1. Genetic variation among individuals within a population provides a mechanistic basis for plasticity and adaptability, such that a multiplicity of genotypes (genetic diversity) provides a greater range of possible functional responses (physiological versatility), and thus a higher probability that a population will resist, or recover from, a perturbation (ecological resilience)2,3. Despite an advanced conceptual understanding of the implied positive relationships among genetic diversity, physiological versatility and ecological resilience, empirical evidence for their existence is lacking from natural populations, and strongly biased towards experiments on model organisms and clonal plants4. Knowledge about the role of genetic diversity in underpinning species performance and ecosystem vulnerability is, however, critical to successfully mitigate impacts of pressures, such as population over-exploitation, pollution, invasive species and global warming.
Increasing temperatures have already impacted most ecosystems on the planet5–7. The mechanisms that translate the physical forcing of climate change into biological changes are, however, poorly understood, creating major uncertainty about future ecological scenarios8,9, and limiting our ability to predict impacts9 and implement mitigation strategies, such as targeted conservation and rehabilitation10,11. A critical knowledge gap relates to the response of species to impending changes, and the role of genetic factors in mediating population persistence through latent functional responses4,9,12. That is, the resilience of a population to climatic stress might depend on possessing sufficient genetic variation to allow a range of responses to stressors, some of which will promote population persistence.
Our lack of understanding of how genetic diversity mediates population response to climate stress is particularly acute for ‘foundation species’ (trees, corals, kelps, etc.)4,12, because of their critical influence on community organization and ecosystem functioning. Indeed, the importance of foundation species in mediating climate stress for associated biodiversity is likely to increase in a warmer future2,13. Where populations of foundation species have reduced potential to respond to environmental stress, resilience might be compromised14 and, if perturbations are severe, entire populations could perish with impacts cascading through the ecosystem15,16. Knowledge of the role of genetic diversity in determining the response of foundation species to climatic stress is, therefore, particularly critical for assessing the overall vulnerability of an ecosystem, and to ensure successful conservation and management strategies2,4,11,13,17,18.
Here, we combined population genetics19 with eco-physiological and ecological field experiments14 to examine relationships between latitudinal patterns in genetic diversity, physiological versatility and ecological resilience of kelp forests, one of the most important foundation species of temperate marine habitats globally20,21. We also documented subsequent responses of kelp (Ecklonia radiata) forests to an extreme climatic perturbation (a marine heatwave), thus demonstrating through a ‘natural experiment’, the possible influence of latitudinal patterns in genetic diversity on ecosystem vulnerability to climatic stress.
Results
We measured genetic diversity, physiological versatility and ecological resilience in 12 kelp forests (Ecklonia radiata) along a latitudinal gradient in Western Australia14 (Fig. 1a). E. radiata is a dominant foundation species on reefs throughout temperate Australasia21, where it exerts a critical influence on biodiversity and ecosystem functioning22,23.
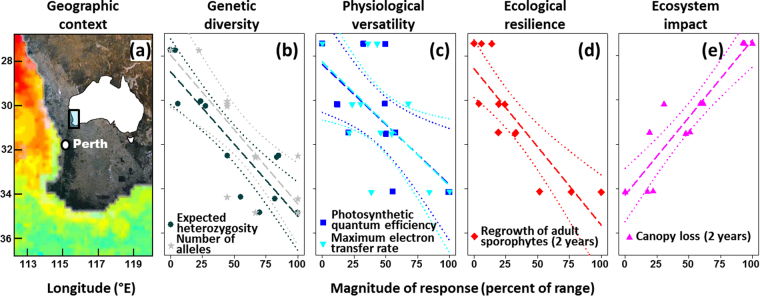
Figure 1.
Geographic context and population characteristics for 12 Australian kelp forests. (a) The coastline of southwestern Australia is swept by the poleward flow of the warm Leeuwin Current, which creates a uniform gradient in ocean temperature of 2–3 °C across latitudes from 27°S to 35°S (annual daily mean 21.9 to 19.5 °C, respectively) (Wernberg & Smale, 2009, see also Fig. S1). Prior to the 2011 marine heat wave, kelp forests had their equatorward limit in Kalbarri (27.7°S). (b) Genetic diversity (data range: He 0.269–0.375, Na 12–21)19, (c) physiological versatility (data range: α 2.8–12.4%, ETRmax 2.3–22.2%) and (d) ecological resilience (data range: 1.2–11.1 kelps m−2)14 of kelp forests measured prior to the heat wave. (e) Ecosystem impact (data range: −86–26% change in kelp forest cover) of the 2011-extreme heat wave measured two years after the event. Latitude is shown on the y-axis for all panels and scaled population characteristics on the x-axis for panels (b–e). Lines represent linear regressions (dashed) with associated 95% confidence limits (dotted). Regression coefficients are given in Table S1. The map (Fig. 1a) was generated in Google Earth version 7.1.8.3036 (https://www.google.com/earth/; © CNES/SpotImage, Data SIO, NOAA, US Navy, NGA, GEBCO) and modified using GIMP version 2.8.14 (https://www.gimp.org/). This included drawing and adding the insert map of Australia.
We found a strong, positive, relationship between latitude and genetic diversity of kelp, measured as both expected heterozygosity (r2 = 0.74, P = 0.002) and number of alleles (r2 = 0.58, P = 0.007), across southwestern Australia (Fig. 1b, see appendix S1 for additional regression statistics). We also found significant, positive, relationships between latitude and physiological versatility (quantum efficiency: r2 = 0.44, P = 0.037; maximum electron transfer rate: r2 = 0.36, P = 0.041; Fig. 1c) and ecological resilience (r2 = 0.68, P = 0.009, Fig. 1d). That is, compared to kelp forests at high (cool) latitudes, kelp forests at low (warm) latitudes had less genetic variation, a narrower range of physiological responses to changes in canopy cover, and suppressed regrowth following experimental canopy loss.
Subsequently, these 12 kelp forests experienced an extreme marine heatwave, where ocean temperatures along 2,000 km of coastline soared above anything seen for at least 140 years24,25. The latitudinal patterns of genetic diversity, physiological versatility, and ecological resilience were mirrored in kelp forest response to the heatwave (r2 = 0.84, P < 0.001; Fig. 1e), with strikingly different impacts observed across the 12 kelp forests (Figs 1e, 2). Despite all forests experiencing similar monthly climatological maximum anomalies during the five months capturing the heatwave (appendix S2), suggesting a similar magnitude of deviation from the norm, low latitude forests with the lowest genetic diversity disappeared completely (Fig. 2a), whereas there were no discernible changes in high latitude forests with the highest genetic diversity (Fig. 2c). Kelp forests at mid latitudes showed partial canopy loss (Fig. 2b).
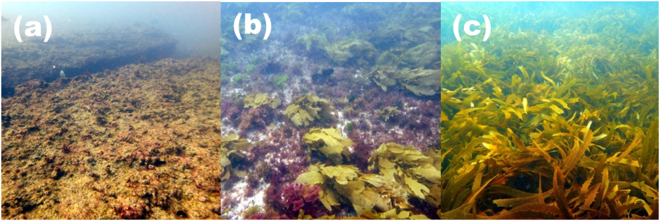
Figure 2.
Impact of the 2011 marine heat wave on kelp forests with different genetic diversities. During the Austral summer of 2011, an extreme marine heat wave devastated low latitude kelp forests with low genetic diversity (a), whereas forests with intermediate genetic diversity showed partial kelp canopy loss (b) and high latitude high-diversity forests showed no discernible impact on kelp canopy cover (c) despite similar temperature anomalies (Fig. S2). Prior to the 2011 marine heat wave, there were no differences in kelp canopy cover among these kelp forests14. All photos taken by T. Wernberg.
To investigate possible drivers of these patterns, we compared the contribution of several potential physical and biological predictors (Table 1, appendix S3). Genetic diversity was the only one to consistently rank as the top predictor of heatwave impact (Table 1) being selected in all of the five top performing (lowest AICc) combinations of predictors (Table 1). In contrast, the heatwave alone (measured as cumulative monthly climatological maximum anomalies ~ sum of temperatures exceeding the long-term maximum for each region26), explained little variation in impact and was only selected (with genetic diversity) in one of the five top performing combinations of predictors (Table 1).
Table 1.
Distance-based linear modelling relating physical and biological predictors (appendix S3) of kelp forest responses to the heatwave.
| Marginal tests | ||||
|---|---|---|---|---|
| Predictor variable | SS(trace) | Pseudo-F | P | Variation explained |
| Genetic diversity (He) | 13334 | 58.00 | 0.0002 | 85.3% |
| Heatwave | 4732 | 4.34 | 0.07 | 30.2% |
| Nutrient concentration | 4059 | 3.51 | 0.09 | 26.0% |
| Reef topography | 1607 | 1.15 | 0.31 | 10.3% |
| Turf/foliose seaweeds | 1090 | 0.75 | 0.40 | 7.0% |
| Wave exposure | 788 | 0.53 | 0.48 | 5.0% |
| Fish herbivores | 165 | 0.11 | 0.76 | 1.1% |
| Depth | 43 | 0.03 | 0.87 | 0.3% |
| Best model selection | ||||
| AICc | r 2 | Predictor variables | ||
| 68.4 | 0.85 | Genetic diversity | ||
| 69.6 | 0.88 | Genetic diversity, Reef topography | ||
| 70.3 | 0.87 | Genetic diversity, Heatwave | ||
| 70.9 | 0.87 | Genetic diversity, Nutrient concentration | ||
| 71.0 | 0.87 | Genetic diversity, Turf/foliose seaweeds | ||
Top half: Marginal tests ascertaining the relationships to individual predictors (total trace = 15633). Bottom half: Multiple regression to ascertain the best (lowest AICc) combinations of predictors, showing the five best models overall.
Discussion
Current understanding of how genetic diversity mediates ecosystem vulnerability to climatic stress has a strong theoretical basis, but evidence from natural populations is rare. Here, we provided empirical evidence for strong covariation between functional responses and population vulnerability to environmental stress, and latitudinal patterns in genetic diversity of kelp. These insights were only possible through a unique combination of independent broad scale field studies, controlled manipulations, and a ‘natural disturbance experiment’ affecting an entire coastline. Given the opportunistic and correlative nature of events and data collections, our study was not designed to tease apart the exact causal mechanisms linking latitudinal patterns of genetic variation, physiological and ecological performance, and population impact. Moreover, identifying underlying causes of these patterns is challenging, because latitudinal gradients often integrate multiple mechanisms, many of which are not mutually exclusive, and can covary themselves. However, even if significant association between predictors and responses do not prove cause-effect relationships per se (e.g.27), such observations remain critical as a foundation for subsequent detailed experiments to pin-point processes. Moreover, because other studies have found limited outcrossing to strongly reduce kelp sporophyte fecundity and fitness28, and linked kelp survival and regrowth after canopy loss to variation in photo-physiology29,30, there exists a plausible mechanistic link between genetic diversity, physiological performance and canopy persistence in the face of disturbance. This link should underpin future studies designed to definitively demonstrate causality.
Kelp forests are generally robust to disturbances, as canopies can recover through recruitment of new gametophytes31, from surviving ‘seed banks’ of microscopic stages that can persist for many months under unfavorable conditions32, or from macroscopic sporophytes resisting the perturbation29. Nevertheless, our experiments prior to the heatwave revealed systematic differences in the physiology and capacity for recovery and regrowth across the 12 kelp forests, matching the latitudinal patterns of genetic diversity. This is consistent with the hypothesis that the initially observed differences in impact of the heatwave were not caused by an external disturbance alone (e.g., the heatwave itself). Nevertheless, subsequent trajectories of recovery could have been influenced by post-disturbance forces such as Allee effects33, or concurrent changes in consumer pressure34. Almost six years have now elapsed without any signs of recovery in the most heavily impacted populations25. No macroscopic sporophytes have been observed (Wernberg & Bennett, personal observation). Because ‘seedbank’ longevity is thought to be less than one year32, and new zoospores would have to arrive against flow of the predominant current15, it seems unlikely these kelp forests will recover in the near future.
Several mechanisms could explain the differences in genetic diversity among kelp forests along the latitudinal gradient and have implications for interpreting the response of kelp forests to the heatwave. These include (i) reduced connectivity or smaller effective population sizes in marginal populations19,35–38, (ii) geometric constraints on the distribution of genotypes (a ‘mid-domain effect’39), (iii) selection for a narrow subset of stress-tolerant genotypes at low latitudes35,40,41, or (iv) historical extinction or colonization events42,43. These mechanisms are not mutually exclusive and might occur simultaneously. For example, selection for stress tolerant genotypes and restricted connectivity are both likely to be important where a population is contracting from deteriorating environmental conditions at its range edge (e.g., shifting isotherms35,41). Teasing apart the mechanism behind the observed patterns of genetic diversity is beyond the scope and capacity of the present study, however, given that the response of low latitude kelp forests to the marine heatwave (extirpation) was opposite to what one would expect for stress-tolerant genotypes (persistence), it is unlikely that selection was the primary mechanism driving the observed patterns of genetic diversity44.
We propose that latitudinal variation in genetic diversity may have played a role in mediating the response of kelp forests to the marine heatwave. First, high genetic diversity coincided with slightly more benign (cooler) pre-heatwave conditions, possibly protecting these kelp forests against change (Fig. S4c, “H”, open circle), whereas low diversity forests experienced warmer initial conditions, predisposing them to an abrupt population crash following the heat wave (Fig. S4c, “K”, open circle). Thus, despite both pre-heatwave temperature and genetic diversity covarying among populations and likely being important in determining kelp response, differences among initial temperature conditions alone cannot fully explain the observed impact to kelp forests. The partial mortality of populations within six of the observed sites – characterized by intermediate genetic diversity – highlights the high variability in stress tolerance among individual kelp plants. If within-population genetic differences in stress tolerance did not play a role in driving the observed impacts, then a more pronounced threshold response to the heatwave would have been expected in all kelp forests, irrespective of genetic diversity, with very low and high impacts, below and above the temperature threshold, respectively. The differences in population persistence following the heatwave suggest that while factors such as initial temperature conditions and absolute magnitude of the anomaly might have contributed to the observed impacts (Fig. S4c,f), variability in stress tolerance among different genotypes, as well as the proportion of genotypes in each population, are likely to have contributed to the observed responses.
We suggest that low diversity populations represent a subset of genotypes created via processes such as low connectivity and effective population sizes36 rather than prior selection for thermal stress (Fig. S4), where these populations would instead represent a subset of genotypes with higher tolerance to warmer ambient conditions. If the latter had been the case, low diversity, but better adapted populations, would have had higher persistence during the heat wave; this was contrary to the observed changes. We cannot rule out the possibility of some local thermal adaptation within populations, as has been observed in a range of organisms (e.g.45,46). Indeed, in the wake of the population impacts observed here, it is conceivable that genotypic frequencies, or population tolerances46, now reflect selection, with only tolerant genotypes surviving (but see Pearson et al.44). Although this study cannot establish causal links it strongly suggests that genetic diversity may at least partially explain the dramatic differences in observed population impacts.
The relationships among genetic, physiological, and ecological population-level responses of kelp forests occurred over a temperature gradient of similar magnitude to projected sea temperature increases in the upcoming 50–100 years10. It is therefore plausible that impending environmental changes will cause substantial ecological changes in temperate marine ecosystems, and that patterns of genetic diversity could play a role in mediating the manifestations of these impacts. Importantly, our data provides an empirical example of how functional responses and vulnerability to environmental stress might be anticipated by mapping population level genetic diversity. This result forms the basis for future work establishing general genetic diversity relationships that are independent of latitude and may transcend geographic settings and stressors. Establishing such predictive relationships of genetic diversity for conferring physiological versatility and ecological resilience is particularly critical for foundation species, because they exert an essential influence on biodiversity and energy flow across multiple trophic levels2,4,13. Moreover, establishing such relationships is vital to assessing the vulnerability of populations9, especially where genetic diversity might already be low4,35 or exclusively maintained in refugia38,47,48, where future exploitation37 or climatic forcing is predicted to be greatest49 and where normal mechanisms of recovery might be compromised (e.g., limited propagule supply or invasion of consumers15,17,50,51). Loss of genetic diversity could drive widespread loss of physiological versatility and ecological resilience, with flow-on effects cascading through to critical, and potentially irreversible, changes to ecosystem structure and functioning.
Methods
We examined potential drivers of ecosystem vulnerability to climate stress by comparing baseline datasets that compared latitudinal patterns in genetic diversity, physiology and resilience, to the response of kelp forest ecosystems to a natural climatic event. Ecklonia radiata is the only true Laminarian kelp along this coastline so the term “kelp” refers to Ecklonia radiata throughout this manuscript. However, Ecklonia radiata forms both monospecific52 and mixed forests with fucoids53 and here we sampled monospecific forests or, for canopy removal experiments, those comprised of at least 50% Ecklonia radiata cover.
Baseline data on physiological versatility and ecological resilience, as well as subsequent population persistence following an extreme climatic perturbation (a marine heatwave), were measured in 12 kelp forests (Ecklonia radiata) along a latitudinal gradient in Western Australia14, Fig. 1a. We sampled three kelp forests (>1 km apart) within each of four regions (>250 km apart), extending ~7° latitude poleward from the warm range-edge of kelps in southwestern Australia (Fig. 1a). Physiological versatility was measured in 2006 and ecological resilience between 2006–200814. The response of the same kelp forests to the heatwave which occurred in 201126, was measured after the event in 201354. Baseline genetic diversity was estimated using kelp plants collected along the same latitudinal gradient and locations, but slightly different sites (a few km apart) in 200619. Genetic diversity was thus matched to each exact kelp forest using its strong relationship with latitude (Table S2). Given the patterns of isolation by distance along this coastline19 this approach is justified.
Genetic diversity was measured as expected heterozygosity (He) and number of alleles (Na), two of the most commonly reported diversity metrics e.g.4, using 6 proven microsatellite markers, in 12 populations, 32 kelps per population19,55. Although these are neutral markers that do not enable firm inferences about the effects of genetic diversity on selection or adaptation, there is evidence that neutral marker diversity can be positively correlated with key demographic parameters of population performance, such as reproductive success56 and survival of juveniles and adults57–59. Importantly, these markers can demonstrate key attributes of breeding systems, such as gene flow and the level of population isolation, factors which correlate with fitness and evolutionary potential60. Indeed, in Western Australia E. radiata populations are characterized by strong isolation by distance driven by poleward flowing boundary currents19,61,62.
Physiological versatility was measured as the coefficient of variation (standard error divided by the mean) in photosynthetic performance (see below for details) of kelp recruits (8–10 kelps per population) 80 days after experimental kelp canopy removal14. The coefficient of variation was used because it provides an estimate of variation in responses within each population, unbiased by potential absolute differences in light climate or physiological performance, and thus links with our hypothesis that greater genetic diversity confers a greater range of responses to stress. The canopy removal treatment mimicked localized canopy loss, a stressor which is predicted to escalate as climate change drives increasingly severe heatwaves, storms, and shifts the distribution and abundance of major herbivores10,50. Photosynthetic quantum efficiency (α) and maximum electron transfer rate (ETRmax) of photosystem two (PSII) were measured by Pulse Amplitude Modulated [PAM] fluorometry after 15 minutes of dark-adaption14. While these measurements were instantaneous, the treatment responses integrated the capacity for long-term (80 days) acclimation to canopy loss. Photosynthesis is a fundamental metabolic process in seaweeds and variation in photosynthetic performance, including quantum efficiency, has been linked to differences in kelp survival and kelp canopy recovery in the face of perturbation, presumably as a consequence of differences in tolerance to excessive light and photoinhibition29,63. Moreover, photosynthetic performance has been directly linked to climatic perturbation in both juvenile14,64 and adult65,66 E. radiata.
Ecological resilience of kelp forests was measured as kelp canopy re-growth two years after experimental kelp canopy removal (mean density of re-established adult kelps in six plots of complete canopy removal per population14). Our measurements of kelp canopy re-growth do not differentiate between micro- and macroscopic kelp recruits surviving in the understory, and new recruitment from surrounding adults. This measure of resilience, therefore, integrates elements of both resistance to, and recovery from, disturbances.
Population impacts in response to an extreme natural climatic perturbation – a marine heatwave (appendix S2) - were assessed from changes in landscape-scale cover of kelp forests surveyed in 2005 (prior to the heatwave), and again in 2013 (after the heatwave). For each kelp forest, the canopy cover was determined along ten haphazardly positioned, non-overlapping, 25 m transects, first in 200552 and then again in 2013. Regular visits to all kelp forests between 2005 and December 2010 (the onset of the event) showed no visible changes to kelp cover in the period prior to the heatwave67, T. Wernberg & S. Bennett personal observation.
To allow better direct visual comparisons of all response variables across latitudes, the magnitude of response in each population characteristic was scaled as a percentage of the range across all 12 kelp forests. However, the range of raw values for each population characteristic are given in the Fig. 1 legend. The strength of relationships between latitude and genetic diversity (He and Na), physiological versatility, resilience and heatwave impact were tested with linear mixed models using R v.3.2.2. For these analyses, the nlme package was used to model data with ‘Region’ included as a random factor to account for underlying spatial autocorrelation among sites (n = 12). The r2 from the linear mixed model was calculated using the sem.model.fits function based on the marginal r2 formula of Nakagawa and Schielzeth68. This marginal r2 quantifies the proportion of total variability that is explained by the fixed effects terms in the model.
Distance-based linear modeling and redundancy analysis was used to identify the relative strength of relationships between population impacts following the heatwave, and seven potential physical and biological predictor variables (appendix S3). Because genetic diversity (Fig. 1b) was sampled independently of the other population characteristics i.e. at slightly different sites (Fig. 1c-e), genetic diversity was attributed to each exact kelp forest using its strong relationship with latitude (Table S2). Thus, it was not possible to include latitude as a predictor in the models because of its correlation to genetic diversity. More importantly, however, latitude does not carry any mechanistic process in itself but is a proxy for processes that may vary along the same scales69. Instead of using a proxy, we used a range of measured site characteristics that are widely known to influence kelp forests (e.g. nutrients, depth, herbivory, wave exposure, reef topography, cf. Table S2). Given He and Na covary across latitude we chose to use only He as a measure of genetic diversity in these analyses. Based on geometric (Euclidian) distances between samples, these analyses are analogous to standard multiple regressions70, where significance is obtained through a randomization test (here 9999 permutations of residuals under a reduced model), thus avoiding assumptions about normal distribution of data and residuals (for technical details, please refer to70). To identify which predictor combinations best explained variation in kelp forest responses to the heatwave, we used a model selection procedure with an exhaustive search among all predictor combinations and a distance-based analogue to the Akaike’s Information Criterion, modified to accommodate small sample sizes relative to the number of predictor variables (AICc). This procedure identified the most parsimonious (lowest AICc) predictor subsets see70, for, technical, details, while also taking co-linearity among predictors (r2 < 0.42, Fig. S3) into account71. To be conservative, we interpreted these analyses qualitatively because 12 sites (albeit large replication in a field setting) provides low power to quantitatively partition variation among sets of multiple predictor variables.
Electronic supplementary material
Acknowledgements
Funded by the Australian Research Council through Discovery (TW, MC) and Future Fellow (TW) grants with additional funding from the Hermon Slade Foundation (TW, SB). MST was supported by the Marsden Fund of New Zealand and FT by the ‘Ramón y Cajal’ program from the Spanish Government. J. Wright, G. Kendrick and S. H. Brawley commented on versions of the manuscript. All data is archived with the authors and is available upon request.
Author Contributions
T.W. and M.C. conceived the idea. T.W., M.C., S.B., M.T., F.T. and B.K. collected and analysed the data. M.C. and T.W. wrote the manuscript and T.W., M.C., S.B., M.T., F.T. and B.K. edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Thomas Wernberg and Melinda A. Coleman jointly supervised this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20009-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Darwin, C. On the Origin of Species by Means of Natural Selection, or, The Preservation of Favoured Races in the Struggle for Life, John Murray, Albemarle Street, London. pp. 502 (1859).
- 2.Reusch TBH, Ehlers A, Hammerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences. 2005;102:2826–2831. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes AR, Stachowicz JJ. Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8998–9002. doi: 10.1073/pnas.0402642101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes AR, Inouye BD, Johnson MTJ, Underwood N, Vellend M. Ecological consequences of genetic diversity. Ecology Letters. 2008;11:609–623. doi: 10.1111/j.1461-0248.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- 5.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 6.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 7.Pecl, G. T. et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science355, 10.1126/science.aai9214 (2017). [DOI] [PubMed]
- 8.Planque B, Bellier E, Loots C. Uncertainties in projecting spatial distributions of marine populations. ICES Journal of Marine Science: Journal du Conseil. 2011;68:1045–1050. doi: 10.1093/icesjms/fsr007. [DOI] [Google Scholar]
- 9.Valladares F, et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecology Letters. 2014;17:1351–1364. doi: 10.1111/ele.12348. [DOI] [PubMed] [Google Scholar]
- 10.Wernberg T, et al. Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. Journal of Experimental Marine Biology and Ecology. 2011;400:7–16. doi: 10.1016/j.jembe.2011.02.021. [DOI] [Google Scholar]
- 11.Magris RA, Pressey RL, Weeks R, Ban NC. Integrating connectivity andclimate change into marine conservation planning. Biological Conservation. 2014;170:207–221. doi: 10.1016/j.biocon.2013.12.032. [DOI] [Google Scholar]
- 12.Hoffmann AA, Sgro CM. Climate change and evolutionary adaptation. Nature. 2011;470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- 13.Halpern BS, Silliman BR, Olden JD, Bruno JP, Bertness MD. Incorporating positive interactions in aquatic restoration and conservation. Frontiers in Ecology and the Environment. 2007;5:153–160. doi: 10.1890/1540-9295(2007)5[153:IPIIAR]2.0.CO;2. [DOI] [Google Scholar]
- 14.Wernberg T, et al. Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecology Letters. 2010;13:685–694. doi: 10.1111/j.1461-0248.2010.01466.x. [DOI] [PubMed] [Google Scholar]
- 15.Smale D, Wernberg T. Extreme climatic event drives range contraction of a habitat-forming species. Proceedings of the Royal Society B. 2013;280:20122829. doi: 10.1098/rspb.2012.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen MS, et al. Habitat Cascades: The Conceptual Context and Global Relevance of Facilitation Cascades via Habitat Formation and Modification. Integr. Comp. Biol. 2010;50:158–175. doi: 10.1093/icb/icq042. [DOI] [PubMed] [Google Scholar]
- 17.Coleman, M. A. et al. Anticipating changes to future connectivity within a network of marine protected areas. Global Change Biology, n/a-n/a, 10.1111/gcb.13634 (2017). [DOI] [PubMed]
- 18.Gerstenmaier CE, Krueger-Hadfield SA, Sotka EE. Genotypic diversity in a non-native ecosystem engineer has variable impacts on productivity. Marine Ecology Progress Series. 2016;556:79–89. doi: 10.3354/meps11809. [DOI] [Google Scholar]
- 19.Coleman MA, et al. Variation in the strength of continental boundary currents determines continent-wide connectivity in kelp. Journal of Ecology. 2011;99:1026–1032. doi: 10.1111/j.1365-2745.2011.01822.x. [DOI] [Google Scholar]
- 20.Steneck RS, et al. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation. 2002;29:436–459. doi: 10.1017/S0376892902000322. [DOI] [Google Scholar]
- 21.Bennett S, et al. The ‘Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests. Marine and Freshwater Research. 2016;67:47–56. doi: 10.1071/MF15232. [DOI] [Google Scholar]
- 22.Ling S. Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia. 2008;156:883–894. doi: 10.1007/s00442-008-1043-9. [DOI] [PubMed] [Google Scholar]
- 23.Coleman MA, Vytopil E, Goodsell PJ, Gillanders BM, Connell SD. Diversity and depth-related patterns of mobile invertebrates associated with kelp forests. Marine and Freshwater Research. 2007;58:589–595. doi: 10.1071/MF06216. [DOI] [Google Scholar]
- 24.Wernberg T, et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nature Climate Change. 2013;3:78–82. doi: 10.1038/nclimate1627. [DOI] [Google Scholar]
- 25.Wernberg T, et al. Climate-driven regime shift of a temperate marine ecosystem. Science. 2016;353:169–172. doi: 10.1126/science.aad8745. [DOI] [PubMed] [Google Scholar]
- 26.Hobday AJ, et al. A hierarchical approach to defining marine heatwaves. Progress in Oceanography. 2016;141:227–238. doi: 10.1016/j.pocean.2015.12.014. [DOI] [Google Scholar]
- 27.Hawkins BA. Eight (and a half) deadly sins of spatial analysis. Journal of Biogeography. 2012;39:1–9. doi: 10.1111/j.1365-2699.2011.02637.x. [DOI] [Google Scholar]
- 28.Raimondi PT, Reed DC, Gaylord B, Washburn L. Effects of self-fertilisation in the giant kelp, Macrocystis pyrifera. Ecology. 2004;85:3267–3276. doi: 10.1890/03-0559. [DOI] [Google Scholar]
- 29.Toohey BD, Kendrick GA. Survival of juvenile Ecklonia radiata sporophytes after canopy loss. Journal of Experimental Marine Biology and Ecology. 2007;349:170–182. doi: 10.1016/j.jembe.2007.05.008. [DOI] [Google Scholar]
- 30.Fejtek SM, Edwards MS, Kim KY. Elk Kelp, Pelagophycus porra, distribution limited due to susceptibility of microscopic stages to high light. Journal of Experimental Marine Biology and Ecology. 2011;396:194–201. doi: 10.1016/j.jembe.2010.10.022. [DOI] [Google Scholar]
- 31.Reed DC, Schroeter SC, Raimondi PT. Spore supply and habitat availability as sources of recruitment limitation in the giant kelp Macrocystis pyrifera (Phaeophyceae) Journal of Phycology. 2004;40:275–284. doi: 10.1046/j.1529-8817.2004.03119.x. [DOI] [Google Scholar]
- 32.Hoffmann AJ, Santelices B. Banks of Algal Microscopic Forms Hypotheses On Their Functioning and Comparisons With Seed Banks. Marine Ecology Progress Series. 1991;79:185–194. doi: 10.3354/meps079185. [DOI] [Google Scholar]
- 33.Bennett S, et al. Canopy interactions and physical stress gradients in subtidal communities. Ecology Letters. 2015;18:636–645. doi: 10.1111/ele.12440. [DOI] [PubMed] [Google Scholar]
- 34.Bennett S, Wernberg T, Harvey ES, Santana-Garcon J, Saunders B. Tropical herbivores provide resilience to a climate mediated phase-shift on temperate reefs. Ecology Letters. 2015;18:714–723. doi: 10.1111/ele.12450. [DOI] [PubMed] [Google Scholar]
- 35.Hampe A, Petit RJ. Conserving biodiversity under climate change: the rear edge matters. Ecology Letters. 2005;8:461–467. doi: 10.1111/j.1461-0248.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- 36.Assis J, et al. High and Distinct Range-Edge Genetic Diversity despite Local Bottlenecks. PLoS ONE. 2013;8:e68646. doi: 10.1371/journal.pone.0068646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robuchon M, Le Gall L, Mauger S, Valero M. Contrasting genetic diversity patterns in two sister kelp species co-distributed along the coast of Brittany, France. Molecular Ecology. 2014;23:2669–2685. doi: 10.1111/mec.12774. [DOI] [PubMed] [Google Scholar]
- 38.Assis J, et al. Major shifts at the range edge of marine forests: the combined effects of climate changes and limited dispersal. Scientific Reports. 2017;7:44348. doi: 10.1038/srep44348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colwell RK, Lees DC. The mid-domain effect: geometric constraints on the geography of species richness. Trends in Ecology & Evolution. 2000;15:70–76. doi: 10.1016/S0169-5347(99)01767-X. [DOI] [PubMed] [Google Scholar]
- 40.Frankham R. Stress and adaptation in conservation genetics. Journal of Evolutionary Biology. 2005;18:750–755. doi: 10.1111/j.1420-9101.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 41.Saada G, et al. Taking the heat: distinct vulnerability to thermal stress of central and threatened peripheral lineages of a marine macroalga. Diversity and Distributions. 2016;22:1060–1068. doi: 10.1111/ddi.12474. [DOI] [Google Scholar]
- 42.Provan J. The effects of past, present and future climate change on range-wide genetic diversity in northern North Atlantic marine species. Frontiers of Biogeography. 2013;5:60–66. [Google Scholar]
- 43.Martinez EA, Cardenas L, Pinto R. Recovery and genetic diversity of the intertidal kelp Lessonia nigrescens (Phaeophyceae) 20 years after El Nino 1982/83. Journal of Phycology. 2003;39:504–508. doi: 10.1046/j.1529-8817.2003.02191.x. [DOI] [Google Scholar]
- 44.Pearson GA, Lago-Leston A, Mota C. Frayed at the edges: selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. Journal of Ecology. 2009;97:450–462. doi: 10.1111/j.1365-2745.2009.01481.x. [DOI] [Google Scholar]
- 45.Howells EJ, et al. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nature Climate Change. 2012;2:116–120. doi: 10.1038/nclimate1330. [DOI] [Google Scholar]
- 46.Bennett S, Wernberg T, Arackal Joy B, de Bettignies T, Campbell AH. Central and rear-edge populations can be equally vulnerable to warming. Nat Commun. 2015;6:10280. doi: 10.1038/ncomms10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assis J, Lucas AV, Bárbara I, Serrão EÁ. Future climate change is predicted to shift long-term persistence zones in the cold-temperate kelp Laminaria hyperborea. Marine Environmental Research. 2016;113:174–182. doi: 10.1016/j.marenvres.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Lourenço CR, et al. Upwelling areas as climate change refugia for the distribution and genetic diversity of a marine macroalga. Journal of Biogeography. 2016;43:1595–1607. doi: 10.1111/jbi.12744. [DOI] [Google Scholar]
- 49.Hobday AJ, Pecl GT. Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Reviews in Fish Biology and Fisheries. 2014;24:415–425. doi: 10.1007/s11160-013-9326-6. [DOI] [Google Scholar]
- 50.Vergés A, et al. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts . Proceedings of the Royal Society B: Biological Sciences. 2014;281:20140846. doi: 10.1098/rspb.2014.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Provost, E. J. et al. Climate-driven disparities among ecological interactions threaten kelp forest persistence. Global Change Biology, 10.1111/gcb.13414 (2017). [DOI] [PubMed]
- 52.Wernberg T, Thomsen MS, Tuya F, Kendrick GA. Biogenic habitat structure of seaweeds change along a latitudinal gradient in ocean temperature. Journal of Experimental Marine Biology and Ecology. 2011;400:264–271. doi: 10.1016/j.jembe.2011.02.017. [DOI] [Google Scholar]
- 53.Coleman MA, Wernberg T. Forgotten underwater forests: The key role of fucoids on Australian temperate reefs. Ecology and Evolution. 2017;7:8406–8418. doi: 10.1002/ece3.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett, S. Ecological drivers of seaweed canopy resilience along a latitudinal climate gradient PhD thesis, University of Western Australia (2015).
- 55.Dolman G, Coleman M. Characterisation of microsatellite loci in the habitat-forming kelp, Ecklonia radiata (Phaeophyceae, Laminariales) Conservation Genetics. 2009;10:657–660. doi: 10.1007/s10592-008-9603-4. [DOI] [Google Scholar]
- 56.Seddon N, Amos W, Mulder RA, Tobias JA. Male heterozygosity predicts territory size, song structure and reproductive success in a cooperatively breeding bird. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2004;271:1823–1829. doi: 10.1098/rspb.2004.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Da Silva A, et al. Genetic diversity-fitness correlation revealed by microsatellite analyses in European alpine marmots (Marmota marmota) Conservation Genetics. 2006;7:371–382. doi: 10.1007/s10592-005-9048-y. [DOI] [Google Scholar]
- 58.Pini J, Planes S, Rochel E, Lecchini D, Fauvelot C. Genetic diversity loss associated to high mortality and environmental stress during the recruitment stage of a coral reef fish. Coral Reefs. 2011;30:399–404. doi: 10.1007/s00338-011-0718-6. [DOI] [Google Scholar]
- 59.Reed DH, Frankham R. Correlation between Fitness and Genetic Diversity. Conservation Biology. 2003;17:230–237. doi: 10.1046/j.1523-1739.2003.01236.x. [DOI] [Google Scholar]
- 60.Franklin, I. R. In Conservation Biology: An Evolutionary perspective (eds M. E. Soule & B. A. Wilcox) 135-150 (Sinauer, 1980).
- 61.Coleman M, Feng M, Roughan M, Cetina-Heredia P, Connell SD. Temperate shelf water dispersal by Australian boundary currents: Implications for population connectivity. Limnology and Oceanography: Fluids and Environments. 2013;3:295–309. [Google Scholar]
- 62.Coleman MA, Gillanders BM, Connell SD. Dispersal and gene flow in the habitat-forming kelp, Ecklonia radiata: relative degrees of isolation across an east-west coastline. Marine and Freshwater Research. 2009;60:802–809. doi: 10.1071/MF08268. [DOI] [Google Scholar]
- 63.Altamirano M, Murakami A, Kawai H. High light stress in the kelp Ecklonia cava. Aquatic Botany. 2004;79:125–135. doi: 10.1016/j.aquabot.2004.01.011. [DOI] [Google Scholar]
- 64.Xiao X, et al. Sensitivity and Acclimation of Three Canopy-Forming Seaweeds to UVB Radiation and Warming. PLoS ONE. 2015;10:e0143031. doi: 10.1371/journal.pone.0143031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stæhr PA, Wernberg T. Physiological responses of Ecklonia radiata (Laminariales) to a latitudinal gradient in ocean temperature. Journal of Phycology. 2009;45:91–99. doi: 10.1111/j.1529-8817.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 66.Wernberg T, de Bettignies T, Bijo AJ, Finnegan P. Physiological responses of habitat-forming seaweeds to increasing temperatures. Limnology and Oceanography. 2016;61:2180–2190. doi: 10.1002/lno.10362. [DOI] [Google Scholar]
- 67.Tuya F, Wernberg T, Thomsen M. Habitat structure affect abundances of labrid fishes across temperate reefs in south-western Australia. Environmental Biology of Fishes. 2009;86:311–319. doi: 10.1007/s10641-009-9520-5. [DOI] [Google Scholar]
- 68.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution. 2013;4:133–142. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- 69.Hawkins BA, Diniz-Filho JAF. ‘Latitude’ and geographic patterns in species richness. Ecography. 2004;27:268–272. doi: 10.1111/j.0906-7590.2004.03883.x. [DOI] [Google Scholar]
- 70.Anderson, M. J., Gorley, R. N. & Clarke, K. R. PERMANOVA+ for PRIMER: Guide to software and statistical methods. 2nd edition edn, 214 (PRIMER-E Ltd, 2008).
- 71.Graham MH. Confronting multicollinearity in ecological multiple regression. Ecology. 2003;84:2809–2815. doi: 10.1890/02-3114. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.