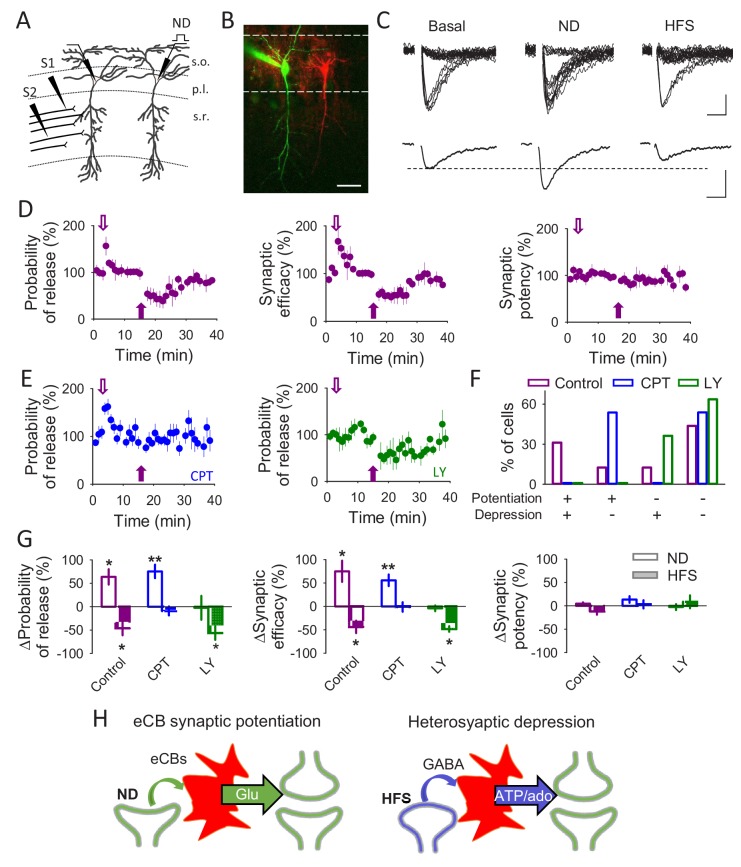
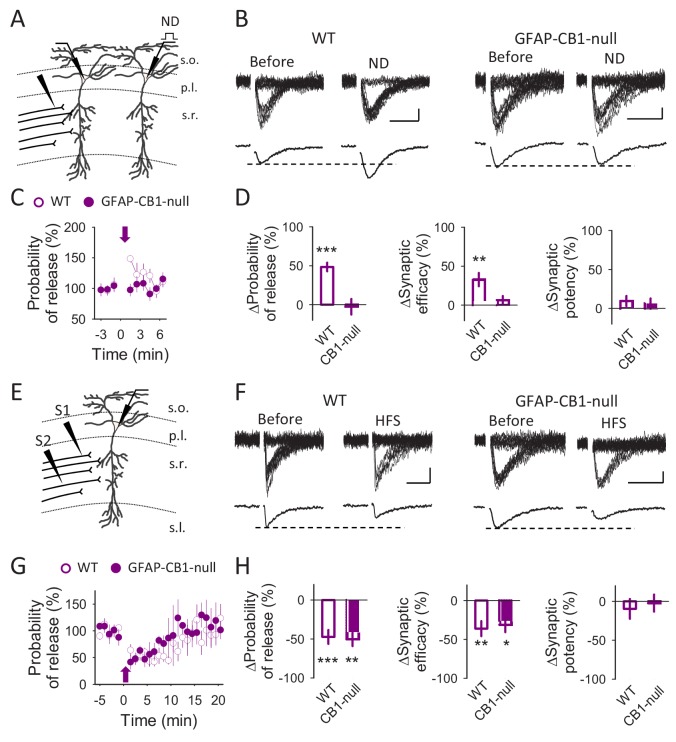
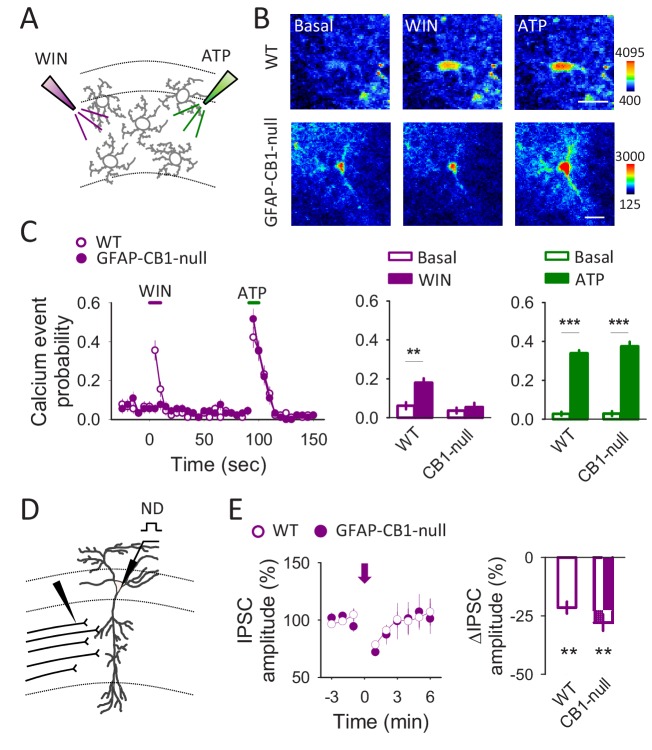
Figure 2. A single synapse can be modulated by different gliotransmitters.
(A) Schematic drawing depicting paired recordings from CA1 pyramidal neurons and the stimulating electrodes (S1 and S2). (B) Fluorescent image showing two pyramidal cells, one loaded with Texas Red (red) and the other one with Alexa 488 (green). Scale bar: 40 µm. (C) Representative EPSC traces (n = 20) and their average traces during basal conditions, after a neuronal depolarization (ND) and after a HFS. Scale bars: 5 pA, 10 ms. (D) Synaptic parameters vs time in control conditions. Open and filled arrows indicate ND and HFS, respectively. (E) Probability of release vs time in the presence of CPT (2 µM) or LY367385 (100 µM). Open and filled arrows indicate ND and HFS, respectively. (F) Percentage of cells that underwent synaptic potentiation, depression, both or no change in the different conditions. (G) Relative changes in synaptic parameters after ND and HFS in control conditions (n = 5) and in the presence of CPT (2 µM; n = 6) or LY367385 (100 µM; n = 4). (H) Schematic summary depicting the signaling pathways leading to eCB-mediated synaptic potentiation or heterosynaptic depression. Data are represented as mean ± s.e.m., *p<0.05, **p<0.01.