Figure 2.
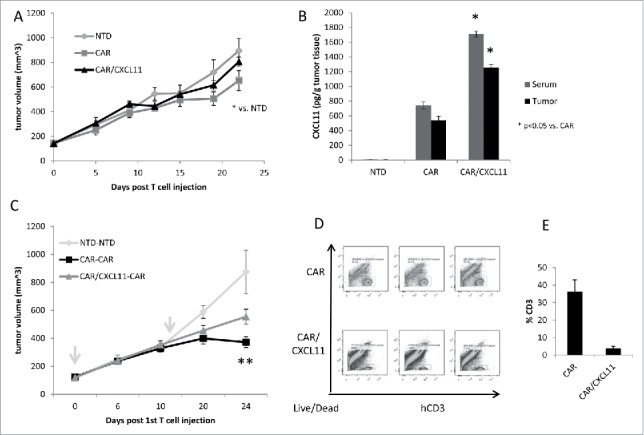
In vivo studies using CAR T cells. A) Tumor-bearing NSG mice were injected intravenously once with 10 million activated T cells that were either non-transduced (NTD), mesothelin-targeted CAR T cells (CAR-GFP), or CAR-CXCL11 T cells. Tumor size was followed over time. Data shown are means ± SEM, n = 5 mice per group. CAR-GFP T cells were significantly smaller than the mice receiving the NTD T cells (* = p < 0.05), with no effect elicited by he CAR-CXCL11 cells. B) On Day 22, tumors were harvested, homogenized and the amount of CXCL11 determined using an ELISA assay. C) In a second experiment, Group 1 animals received one dose of 10 million NTD T cells on Day 0 (arrow) and one dose on Day 11 (arrow). Group 2 mice received one dose of 10 million Meso-CAR T cells on Day 0 and one dose of Meso-CAR T cells on Day 11. Group 3 mice received 10 million CAR-CXCL11 CAR T cells on Day 0 and one dose of 10 million CAR T cells on Day 11. The growth of the tumors was followed until Day 24. Data shown are means ± SEM, n = 7 mice per group. The tumors in the Group 2 mice receiving of two doses of CAR T cells were significantly smaller than the NTD Group 1 mice (p < 0.001). The tumors in the Group 3 mice were significantly smaller than the NTD tumors (p < 0.01), however, the Group 3 tumors were significantly larger than the Group 2 tumors (p < 0.05). D). Tumors were harvested at the end of the study, digested, and the % of live human CD3 T cells measured by flow cytometry. E) Average percent of human CD3 T cells within the CAR vs CAR/CXCL11 groups is plotted. There were significantly more T cells in the CAR group (p < 0.001).
