ABSTRACT
Rice (Oryza sativa L) is one of the most Mn-tolerant crops that can grow in submerged paddy fields, where the Mn concentration in soil solution is very high due to reduction. Although a large part of Mn is transferred from the roots to the shoot in rice, the roots are constantly exposed to high Mn concentrations in submerged paddies. Thus, mechanisms for preventing Mn overaccumulation in the cytoplasm of root cells are necessary. Recently, we showed that two cation diffusion facilitators, MTP8.1 and MTP8.2, play a crucial role in Mn tolerance in rice roots by sequestering Mn in vacuoles. Moreover, we observed that disruption of MTP8.1 and MTP8.2 resulted in reduced Mn accumulation under excess Mn. In the present study, we examined the effects of disruption of MTP8.1 and MTP8.2 on Mn uptake and determined that this phenotype is caused by a rapid and significant reduction of Mn uptake in response to excess Mn. Previously, we showed that Mn export from root cells through MTP9 was promoted by high Mn. Together, these findings suggest that optimal Mn concentration in rice roots is maintained by reduced uptake, vacuolar sequestration, and extrusion by cation diffusion facilitators.
KEYWORDS: Metal tolerance protein, cation diffusion facilitator, transporter, Mn tolerance, Oryza sativa
Mn detoxification is quite an important task for rice growing in submerged paddy fields with low redox potential. It has been demonstrated that rice plants transfer a large part of Mn from the roots to the shoot by the activity of metal tolerance protein 9 (MTP9),1 unload it from xylem at the nodes, and distribute it to old tissues via natural resistance-associated macrophage protein 3 (NRAMP3) in the presence of high Mn concentrations.2 We recently reported that Mn in old leaves is detoxified by two vacuolar transporters, MTP8.1 and MTP8.2.3,4 Both MTP8.1 and MTP8.2 are constitutively expressed at higher levels in old than in young leaves, but the level of MTP8.1 is several times higher than that of MTP8.2.4 Knockout of MTP8.1 resulted in significant growth reduction in addition to chlorosis and necrosis in leaves under high Mn conditions,3 whereas no obvious Mn toxicity symptoms were presented in mtp8.2 leaves.4 However, when MTP8.2 was knocked down in mtp8.1, the mutants (mtp8.2i/mtp8.1) exhibited higher Mn sensitivity than did mtp8.1,4 indicating that MTP8.2 is also important for Mn tolerance in shoots. In roots, MTP8.1 and MTP8.2 were expressed at similar levels regardless of Mn condition.4 MTP8.1 and/or MTP8.2 disruption led to impaired root elongation under excess Mn,4 indicating that vacuolar sequestration by the transporters plays a crucial role in Mn detoxification in roots. Interestingly, the mtp8.2i/mtp8.1 mutants accumulated significantly lower amounts of Mn than did the wild type under excess Mn.4 However, these transporters are not involved in Mn uptake by roots. We hypothesized that rice plants may respond to excess Mn by reducing Mn uptake, thereby enhancing Mn tolerance. In the present study, we present the effect of disruption of MTP8.1/MTP8.2 on Mn uptake and a possible mechanism of Mn tolerance in rice roots.
To examine our hypothesis, we performed a short-term (3-h) uptake experiment that examined whether the MTP8.1/8.2 disruption reduced Mn uptake, according to Ueno et al.5 with some modifications. Seeds of wild-type Nipponbare and mtp8.2i/mtp8.1 plants were germinated in flowing tap water for 4–7 days, and then pre-cultured in 1/2 Kimura B solution with 0.1 μM Mn for 26 days. To investigate Mn uptake, the roots of the seedlings were pre-incubated in 0.5 mM CaCl2 solution for 30 min at either 30°C or 4°C. They were then exposed to 0.5 mM CaCl2 solution containing varying concentrations of Mn (0.5, 2, 10, 50, 100, and 200 μM) for 3 h at 30°C or 4°C. Mn concentrations in roots and shoots obtained at 4°C were subtracted from those at 30°C to determine net root uptake and translocation to the shoot. The results showed that at elevated Mn levels (> 10 μM), mtp8.2i/mtp8.1 exhibited Mn uptake rates that were up to five times lower than in the wild type (Fig. 1A). In contrast, there was no significant difference in Mn translocation to the shoots between the mutant and wild-type plants (Fig. 1B). The inhibition of Mn uptake may be a response from NRAMP5 to compensate for the lack of vacuolar sequestration under MTP8.1/8.2 disruption. NRAMP5 is the major transporter for Mn uptake that localizes in the distal side of the plasma membrane in exodermal and endodermal cells.6 The dramatic increase in Mn in the cytosol of mtp8.2i/mtp8.1 root cells can stimulate the response to Mn toxicity by inhibiting further uptake of Mn through NRAMP5. Because NRAMP5 transcription does not respond to the Mn level,6,7 even in mtp8.2i/mtp8.1 (data not shown), this inhibition may be related to post-transcriptional degradation of NRAMP5. Correlation between Mn tolerance and restriction of Mn uptake has been reported in some plant species. Robson and Loneragan demonstrated that the higher Mn tolerance of subterranean clover than that in toothed medick was attributed to a lower uptake rate.8 In addition, Culvenor noted that Mn tolerance in bulbous canary grass was associated with low Mn uptake.9
Figure 1.
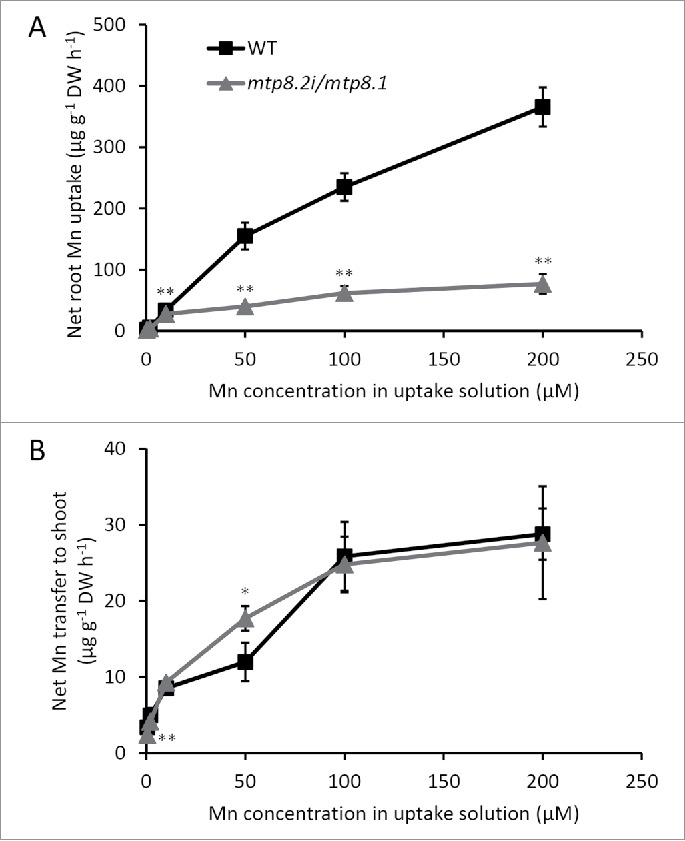
Kinetics of short-term Mn uptake by roots of wild-type (square symbols) and mtp8.2i/mtp8.1 (triangle symbols) plants. Seedlings were exposed to 0.5 mM CaCl2 solution containing varying concentrations of Mn (0.5, 2, 10, 50, 100, and 200 μM) for 3 h at 30°C or 4°C temperatures. Net root Mn uptake (A) and Mn transfer to shoot (B) were calculated by subtracting values at 4°C from those at 30°C. Data represent the mean ± SD (n = 3). **P < 0.01, *P < 0.05, Tukey's test.
In addition to vacuolar sequestration and reduced uptake, Mn concentration in root cells may be controlled by extrusion. The accumulation of Mn exporter MTP9, which is localized in the proximal side of the exodermis/endodermis and mediates Mn uptake in co-ordination with NRAMP5, is promoted in response to Mn.1 However, a similar amount of Mn was transferred to the shoots in mtp8.2i/mtp8.1 and wild type (Fig. 1B), suggesting that this regulation may arise after the onset of NRAMP5 inhibition. The expression of Cucumis sativus MTP9 is upregulated under excess Mn.10 Further, overexpression of CsMTP9 in Arabidopsis thaliana enhanced Mn tolerance in roots. Although accumulation of oxidized Mn in the apoplast is known to be a cause of Mn toxicity,11,12 these studies suggest that extrusion of Mn from cells by MTP9 also mediates Mn tolerance in roots.
The overall evidence suggests that high Mn tolerance in rice roots is associated with stringent control of cytosolic Mn concentration via reduced uptake, constitutive vacuolar sequestration by MTP8.1 and MTP8.2, and enhanced extrusion from cells (Fig. 2). The fast restriction of Mn uptake when exposed to excess Mn may be attributed to post-transcriptional regulation of NRAMP5. Molecular mechanisms for Mn sensing by the roots require further investigation.
Figure 2.
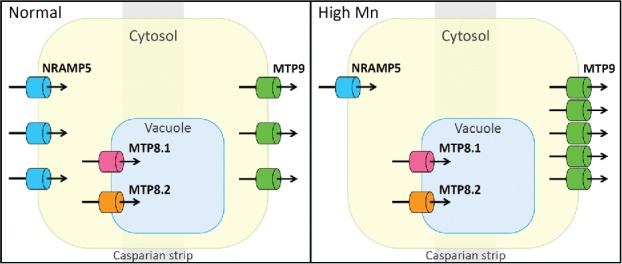
Putative mechanism of Mn tolerance in rice root. Right and left panels show normal and high Mn conditions, respectively. NRAMP5 is localized at the distal side of the exodermis and endodermis, while MTP9 is localized at the proximal side of both layers. MTP8.1 and MTP8.2 are localized to the tonoplast. Optimal Mn concentration in the root may be maintained via reduced uptake caused by the degradation of NRAMP5 and enhanced extrusion from cells caused by an increase in MTP9 accumulation, as well as constitutive vacuolar sequestration by MTP8.1 and MTP8.2.
Funding Statement
This work was supported by Grant-in-Aid for Young Scientist (B) (JSPS KAKENHI Grant Number 15K18660 to D.U.); Specially Promoted Research (JSPS KAKENHI Grant Number 16H06296 to J.F.M.).
Abbreviations
- MTP
metal tolerance protein
- NRAMP
natural resistance-associated macrophage protein
Disclosures
The authors have no conflict of interest to declare.
References
- 1.Ueno D, Sasaki A, Yamaji N, Miyaji T, Fujii Y, Takemoto Y, Moriyama S, Che J, Moriyama Y, Iwasaki K, et al.. A polarly localized transporter for efficient manganese uptake in rice. Nat Plants. 2015;1:15170. doi: 10.1038/nplants.2015.170. [DOI] [PubMed] [Google Scholar]
- 2.Yamaji N, Sasaki A, Xia JX, Yokosho K, Ma JF. A node-based switch for preferential distribution of manganese in rice. Nat Commun. 2013;4:2442. doi: 10.1038/ncomms3442. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Fujii Y, Yamaji N, Masuda S, Yuma T, Kamiya T, Yusuyin Y, Iwasaki K, Kato S, Maeshima M, et al.. Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. J Exp Bot. 2013;64:4375–87. doi: 10.1093/jxb/ert243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takemoto Y, Tsunemitsu Y, Fujii-Kashino M, Mitani-Ueno N, Yamaji N, Ma JF, Kato S, Iwasaki K, Ueno D. The tonoplast-localized transporter MTP8.2 contributes to manganese detoxification in the shoots and roots of Oryza sativa L. Plant Cell Physiol. 2017;58:1573–82. doi: 10.1093/pcp/pcx082. [DOI] [PubMed] [Google Scholar]
- 5.Ueno D, Koyama E, Yamaji N, Ma JF. Physiological, genetic, and molecular characterization of a high-Cd-accumulating rice cultivar, Jarjan. J Exp Bot. 2011;62:2265–72. doi: 10.1093/jxb/erq383. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki A, Yamaji N, Yokosho K, Ma JF. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell. 2012;24:2155–67. doi: 10.1105/tpc.112.096925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishimaru Y, Takahashi R, Bashir K, Shimo H, Senoura T, Sugimoto K, Ono K, Yano M, Ishikawa S, Arao T, et al.. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci Rep. 2012;2:286. doi: 10.1038/srep00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robson AD, Loneragan JF. Sensitivity of annual Medicago species to manganese toxicity as affected by calcium and pH. Aust J Agric Res. 1970;21:223–32. doi: 10.1071/AR9700223. [DOI] [Google Scholar]
- 9.Culvenor RA. Tolerance of Phalaris aquatica L. lines and some other agricultural species to excess manganese, and the effect of aluminium on manganese tolerance in P. aquatic. Aust J Agric Res. 1985;36:695–708. doi: 10.1071/AR9850695. [DOI] [Google Scholar]
- 10.Migocka M, Papierniak A, Kosieradzka A, Posyniak E, Maciaszczyk-Dziubinska E, Biskup R, Garbiec A, Marchewka T. Cucumber metal tolerance protein CsMTP9 is a plasma membrane H+-coupled antiporter involved in the Mn2+ and Cd2+ efflux from root cells. Plant J. 2015;84:1045–58. doi: 10.1111/tpj.13056. [DOI] [PubMed] [Google Scholar]
- 11.Fecht-Christoffers MM, Maier P, Horst WJ. Apoplastic peroxidases and ascorbate are involved in manganese toxicity and tolerance of Vigna unguiculate. Physiol Plant. 2003;117:237–44. doi: 10.1034/j.1399-3054.2003.00022.x. [DOI] [Google Scholar]
- 12.Sasaki A, Yamaji N, Xia J, Ma JF. OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiol. 2011;157:1832–40. doi: 10.1104/pp.111.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]


