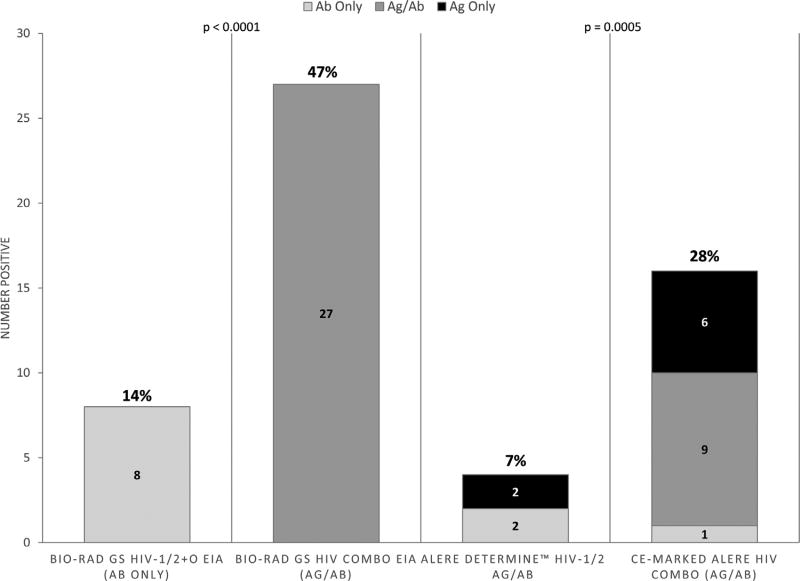
Fig. 3.
Proportion of RNA Positive/3rd Generation Rapid Negative Samples Detected as HIV Positive by Third and Fourth Generation EIA and Fourth Generation Rapid Tests. 57 pre-seroconversion plasma samples with HIV RNA > 20 copies/ml that tested HIV negative using third generation antibody (Ab)-only rapid diagnostic tests were evaluated with Bio-Rad GS HIV-1/2 + O EIA, Bio-Rad HIV Combo Antigen (Ag)/Ab EIA, Alere Determine™ HIV-1/2 Ag/Ab Combo, and Conformité Européene (CE)-Marked Alere™ HIV Combo. 8 of 57 (14%) were positive by Bio-Rad GS HIV-1/2 + O EIA and 27 of 47 (47%) were positive by Bio-Rad HIV Combo Ag/Ab EIA (p < 0.0001). 4 of 57 (7%) were positive by Alere Determine™ HIV-1/2 Ag/Ab Combo (2 Ab only, 2 Ag only, 0 dual Ag/Ab) and 16 of 57 (28%) were positive by CE-Marked Alere™ HIV Combo (1 Ab only, 9 Ag only, 6 dual Ag/Ab).