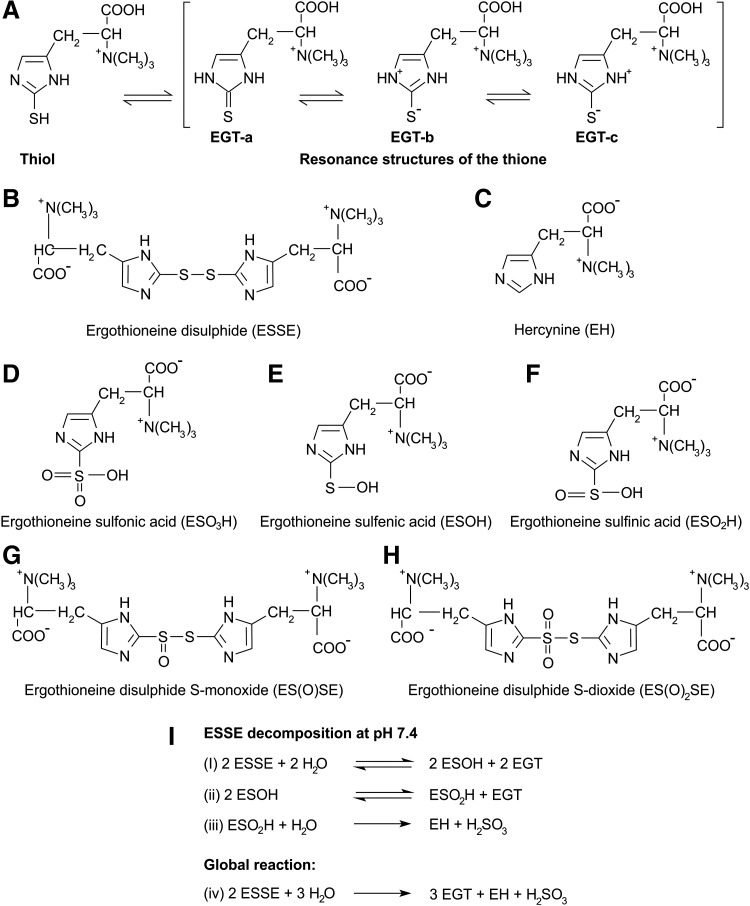
FIG. 2.
Tautomerism of EGT and chemical structures of oxidation states of EGT. (A) EGT exists as a tautomer between its thiol and thione forms. The thione has resonance structures that are thiolate in character and contribute to weak thiol-like activity of EGT. At physiological pH, EGT exists primarily in the thione form, which prevents it from undergoing autoxidation. (B) ESSE is formed when EGT is exposed to hydrogen peroxide, peroxynitrite, or hypochlorite, but due to its instability at physiological pH, it decomposes disproportionately into EGT and hercynine (C). Servillo et al. (57) proposed two oxidation patterns for EGT: (i) EGT is oxidized via the ESSE pathway and in the presence of excess oxidants, ESSE can be transformed into EGT disulfide S-monoxide (G) and EGT disulfide S-dioxide (H); (ii) EGT is first oxidized into EGT sulfenic acid (E), which is further oxidized into EGT sulfinic acid (F) that is oxidized into EGT sulfonic acid (D); Servillo et al. (57) also proposed a pathway for the decomposition of ESSE at neutral pH (I). EGT, ergothioneine; ESSE, ergothioneine disulfide.