Abstract
Hox transcript antisense intergenic RNA (HOTAIR) is a well-known long non-coding RNA (lncRNA) which participates in tumorigenesis and progress of multiple cancers. However, the associations among polymorphisms on HOTAIR, breast cancer (BC) susceptibility and clinical outcomes have remained obscure. In this case-control study, we assessed the interaction between three lncRNA HOTAIR single nucleotide polymorphisms (SNPs) (rs1899663, rs4759314 and rs7958904) on the risk and clinical outcome of breast cancer in a Chinese Han population. In total, 969 breast cancer cases and 970 healthy controls were enrolled in this study. Associations among genotypes, BC risk and survival were evaluated by univariate and multivariate logistic regression to estimate the odds ratio (OR), hazard ratio (HR) and its 95% confidence interval (CI). The disease-free survival (DFS) and overall survival (OS) was calculated by the Kaplan–Meier method. We found that the T allele of rs1899663 and C allele of rs7958904 both achieved significant differences between cases and controls in the single locus analyses (P = 0.017 and 0.010, respectively). Multivariate analyses also revealed the rs1899663 TT genotype and rs7958904 CC genotype were both at higher risk of breast cancer compared with the GG homozygotes (OR = 2.08, 95% CI = 1.20–3.60 and OR = 1.45, 95% CI = 1.01–2.08, respectively). In survival analysis, we observed that the T allele of rs1899663 presented significant differences for both DFS (HR = 1.64, 95% CI = 1.12–2.40) and OS (HR = 2.10, 95% CI = 1.29–3.42) in younger subjects (age ≤ 40). Our findings may provide new insights into the associations among the genetic susceptibility, the fine classifications and the prognosis of breast cancer. Further studies with larger sample size and functional research should also be conducted to validate our findings and better elucidate the underlying biological mechanisms.
Keywords: breast cancer, HOTAIR, polymorphisms, genetic susceptibility, prognosis
INTRODUCTION
Worldwide, breast cancer (BC) is one of the most commonly diagnosed malignancies and the primary cause of death from cancer in women [1]. For the year 2017, it is estimated in the United States that approximately 252,710 female patients would be diagnosed with breast cancer and 40,610 would die from it [2]. During the past few decades in China, the incidence of BC has increased rapidly and become the most frequent cancer for women in major cities [3, 4]. The development of breast cancer is a multifactorial and complex process, involving both environmental and genetic factors. Epidemiology studies have demonstrated that age, obesity, menstrual status, positive family history and previous benign breast disease are correlated with the development of breast cancer [5–10]. Whereas accumulative evidences have revealed that, some genetic variants such as single nucleotide polymorphisms (SNPs) in tumor suppressor genes or oncogenes, could also play a critical role in the genetic susceptibility to breast cancer [11–17]. Although a great proportion of publications have focused on the cancer-related polymorphisms that are located in protein-coding genes, several SNPs located in chromosomal regions which do not encode genes are also indicated to contribute to the risk of different cancers.
In the past few years, one novel kind of non-coding RNAs, long-non coding RNA (lncRNA) has attracted extensive attentions for its wide range and comprehensive regulatory functions in human diseases. LncRNA is a type of RNA transcripts that are longer than 200 nucleotides with no protein-coding capacities [18]. Although lncRNAs were identified to be involved in multiple biological processes [19–22], they were also known to play important roles in tumorigenesis, including transcriptional, post-transcriptional and epigenetic regulation of cancer-associated genes, thereby resulting in the cell progression, migration, invasion and apoptosis [23–25]. As one of these RNAs, lncRNA Hox transcript antisense intergenic RNA (HOTAIR) which is located on chromosome 12q13.13, has been proved to be linked with the development and progression of multiple cancers, such as hepatocellular cancer [26, 27], esophageal cancer [28–30], lung cancer [31–33], gastric cancer [34–37] and breast cancer [38–40]. HOTAIR plays a crucial role in gene regulation by modifying the chromatin structure [41]. The 5′ domain of HOTAIR could bind polycomb repressive complex 2 (PRC2), leading to a histone H3 lysine27 trimethylation (H3K27me3) in the HOXD locus, whereas the 3′ domain connects to the LSD1/CoREST/REST complex with H3 lysine 4 demethylation, together regulating the various downstream genes and promoting cancer cell metastasis [42]. In breast cancer, increasing evidences have suggested that lncRNA HOTAIR is an oncogene which is correlated with the BC carcinogenesis, progression and prognosis. Firstly, aberrant up-regulation of HOTAIR was found in breast cancer tissue or plasma samples compared with normal adjacent non-tumorous tissue or healthy controls [43, 44]. Additionally, this high expression of HOTAIR was also a significant predictor of subsequent metastasis and correlated with a shorter survival time in breast cancer patients [38, 43]. Moreover, in vitro studies have identified that the HOTAIR was robustly expressed in the basal-like breast cancer cells and the inhibition of HOTAIR could reduce the basal-like gene expression and growth [45]. Recently, several single nucleotide polymorphisms located in HOTAIR were also reported to show highly significant associations with breast cancer. For example, one study by Yan et al. [46] identified that the T allele of rs920778 conferred significant increased risk to BC, with the other study in Turkey indicating that the TT genotype of rs12826786 might play critical roles in genetic susceptibility for breast cancer [47]. However, our understanding for the association between lncRNA HOTAIR polymorphisms and the genetic susceptibility of BC is still at an early stage. And as far as we know, no published studies have ever evaluated the relationships between HOTAIR SNPs and the clinical outcomes in breast cancer patients. Accordingly, we selected five SNPs (rs12826786, rs1899663, rs4759314, rs7958904 and rs920778) which were previously identified to be associated with cancer risk and conducted this present case-control study involving 969 BC patients and 970 healthy controls, aiming to investigate the role of HOTAIR tag SNPs on the risk and clinical outcome of breast cancer in a southeast Chinese Han population.
RESULTS
Subject characteristics
A total of 1939 subjects (969 cases and 970 healthy controls) were involved in this study. The selected demographic characteristics and clinicopathological features of breast cancer cases and control subjects are displayed in Table 1. No significant differences were observed between cases and controls in age, menopausal status, age at menopause and previous benign disease (P > 0.05). Compared with the healthy controls, the BC patients were more likely to have a lower mean BMI, an earlier age at menarche, a later age at first live birth and a higher proportion of family history of breast cancer (P < 0.05). Among 969 breast cancer cases, 584 (60.3%) were with tumor size >2 cm, 385 (39.7%) were with tumor size ≤2 cm, 490 (50.6%) patients had lymph node involvement, 479 (49.4%) patients did not have lymph node involvement. Moreover, 644 (66.4%) cases were luminal type, 149 (15.4%) were HER-2 overexpressing and 176 (18.2%) were triple negative breast cancer (TNBC).
Table 1. Basic demographic characteristics and clinical features for breast cancer cases and cancer free-controls.
| Characteristics | Cases (n = 969) no.(%) | Controls (n = 970) no.(%) | P |
|---|---|---|---|
| Age, y (mean ± SD) | 46.9 ± 10.2 | 47.2 ± 11.0 | 0.556 |
| BMI, kg/m2 (mean ± SD) | 22.5 ± 2.6 | 23.1 ± 3.1 | <0.001 |
| Age at menarche, y (mean ± SD) | 15.2 ± 1.7 | 15.5 ± 1.7 | <0.001 |
| Menopausal status | 0.053 | ||
| Premenopausal | 620 | 624 | |
| Postmenopausal | 342 | 327 | |
| Unnatural menopausea | 7 | 19 | |
| Age at menopause, y (mean ± SD) | 50.2 ± 3.0 | 50.3 ± 3.0 | 0.673 |
| Age at first live birth, y (mean ± SD) | 25.0 ± 3.6 | 24.2 ± 3.3 | <0.001 |
| Family history of breast cancer | <0.001 | ||
| Yes | 75 | 12 | |
| No | 894 | 958 | |
| Previous benign breast disease | 0.079 | ||
| Yes | 40 | 26 | |
| No | 929 | 944 | |
| Tumor size | |||
| >2 cm | 584 (60.3) | ||
| ≤2 cm | 385 (39.7) | ||
| Lymph node involvement | |||
| Yes | 490 (50.6) | ||
| No | 479 (49.4) | ||
| Estrogen receptor (ER) status | |||
| Positive | 644 (66.5) | ||
| Negative | 325 (33.5) | ||
| Progestrone receptor (PR) status | |||
| Positive | 566 (58.4) | ||
| Negative | 403 (41.6) | ||
| HER-2 status | |||
| Positive | 315 (32.5) | ||
| Negative | 654 (67.5) | ||
| Molecular subtype | |||
| Luminal type | 644 (66.4) | ||
| HER-2 overexpression | 149 (15.4) | ||
| TNBCb | 176 (18.2) | ||
| Relapse | |||
| Yes | 337 (34.8) | ||
| No | 632 (65.2) | ||
| Death | |||
| Yes | 217 (22.4) | ||
| No | 752 (77.6) |
aUnnatural menopause consists of hysterectomy operation and other status.
bTNBC for triple negative breast cancer.
Effects of HOTAIR SNPs and breast cancer risk
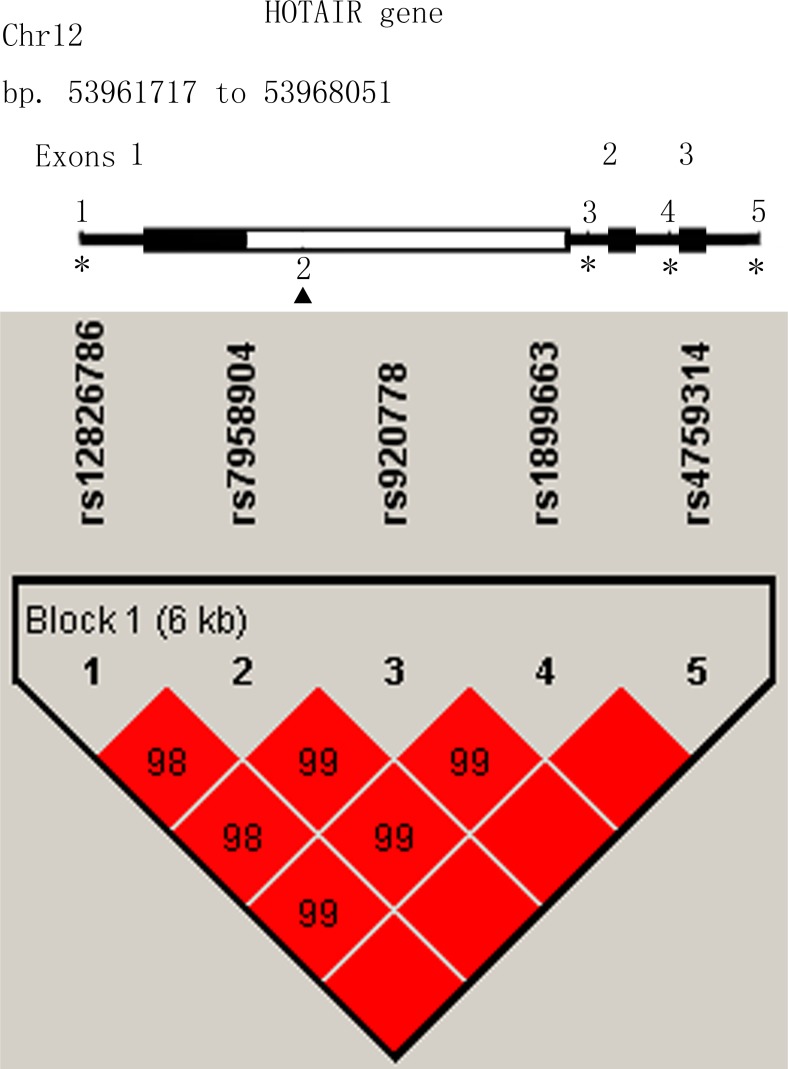
In linkage disequilibrium (LD) analysis, the SNP rs12826786 was discovered in strong LD with rs1899663, with a Pearson’s correlation coefficient (r2) of 0.983. Similarly, the SNP rs920778 was also in strong LD with rs7958904, with a Pearson’s correlation coefficient (r2) of 0.984 (Figure 1). So rs1899663, rs4759314 and rs7958904 were selected as three tag SNPs in this study. The genotype distributions of all three tag SNPs are shown in Table 2. The observed genotype frequencies in three SNPs were consistent with those expected from Hardy-Weinberg equilibrium (HWE) in healthy controls (P = 0.402 for rs1899663, P = 0.295 for rs4759314 and P = 0.764 for rs7958904, respectively). In the single locus analyses, the T allele of rs1899663 and C allele of rs7958904 both achieved significant differences between cases and controls, with the P value of 0.017 and 0.010, respectively. Multivariate logistic regression analyses adjusted by age, BMI, age at menarche, menopausal status and family history of breast cancer also revealed that, for rs1899663 and rs7958904, the TT or CC carriers were both at higher risk of breast cancer compared with the GG homozygotes (OR = 2.08, 95% CI = 1.20–3.60 and OR = 1.45, 95% CI = 1.01–2.08, respectively). However, when we combined the GT and TT genotype of rs1899663, or the GC and CC genotype of rs7958904 to construct a dominant model, no significant increased risk was found. In addition, we didn’t detect any significant correlation for rs4759314 in allelic, co-dominant or dominant model. In the power analysis, we had power of 85.64% and 30.1% to detect an OR of 2.08 (1.20–3.60) and an OR of 1.45 (1.01–2.08) for rs1899663 and rs7958904 for co-dominant model, respectively.
Figure 1. Linkage disequilibrium and genomic location of HOTAIR polymorphisms.
Table 2. Distribution of genotype/allele frequency of three SNPs in HOTAIR and their correlations with breast cancer.
| Genotype | Cases (n = 969) no.(%) | Controls (n = 970) no.(%) | P | Adjusted OR (95% CI)a |
P trendb |
|---|---|---|---|---|---|
| rs1899663 G>T | |||||
| GG | 628 | 665 | 1.00 (Reference) | ||
| GT | 299 | 284 | 0.326 | 1.11 (0.91–1.35) | |
| TT | 42 | 21 | 0.009 | 2.08 (1.20–3.60) | 0.027 |
| GT + TT | 341 | 305 | 0.109 | 1.17 (0.97–1.42) | |
| T allele frequency | 383 (19.8) | 326 (16.8) | 0.017c | ||
| rs4759314 A>G | |||||
| AA | 801 | 817 | 1.00 (Reference) | ||
| GA | 157 | 144 | 0.536 | 1.08 (0.84–1.39) | |
| GG | 11 | 9 | 0.805 | 1.12 (0.45–2.80) | 0.520 |
| GA + GG | 168 | 153 | 0.514 | 1.08 (0.85–1.39) | |
| A allele frequency | 179 (9.2) | 162 (8.4) | 0.338c | ||
| rs7958904 G>C | |||||
| GG | 489 | 537 | 1.00 (Reference) | ||
| GC | 396 | 373 | 0.171 | 1.14 (0.94–1.38) | |
| CC | 84 | 60 | 0.046 | 1.45 (1.01–2.08) | 0.030 |
| GC + CC | 480 | 433 | 0.068 | 1.19 (0.99–1.42) | |
| C allele frequency | 564 (29.1) | 493 (25.4) | 0.010c |
aAdjusted by age, BMI, age at menarche, menopausal status and family history of breast cancer where appropriate.
bP trend for genotypes between cases and controls.
cTwo-sided χ2 test for differences in allele frequency distributions between cases and cancer-free controls.
Stratified analysis of HOTAIR polymorphisms and breast cancer
To further assess the suggestive association between HOTAIR polymorphisms and the risk of breast cancer, we conducted stratified analyses among different subgroups of demographic characteristics and reproductive factors in dominant model (Table 3). For the T carriers of rs1899663, elevated risks of BC were found in subgroups of younger patients (age ≤ 40) (OR = 1.48, 95% CI = 1.04–2.12), individuals with earlier menarche (OR = 1.38, 95% CI = 1.05–1.82) and subjects with an earlier age at first live birth (OR = 1.36, 95% CI = 1.06–1.75). As for the C carriers of rs7958904, we observed significantly increased risks in subgroup of lower BMI individuals (BMI ≤ 24) (OR = 1.26, 95% CI = 1.01–1.57), individuals with an earlier age at first live birth (OR = 1.37, 95% CI = 1.08–1.74) and patients with ER positive (OR = 1.32, 95% CI = 1.07–1.61) or PR positive (OR = 1.32, 95% CI = 1.07–1.64). No positive associations were detected in any of the subgroups of rs4759314 (Supplementary Table 1). Also, no significant heterogeneity was discovered within any of the subgroup for the three tag SNPs.
Table 3. Stratified analysis on associations among rs1899663 and rs7958904 polymorphisms and breast cancer risk.
| Characteristics | rs1899663 | P | OR (95% CI)a | Pb | rs7958904 | P | OR (95% CI)a | Pb | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases ( GG/GT + TT) |
Controls (GG/GT + TT) |
Cases (GG/GC + CC) |
Controls (GG/GC + CC) |
|||||||
| Age | 0.155 | 0.525 | ||||||||
| ≤40 | 164/112 | 192/89 | 0.031 | 1.48 (1.04–2.12) | 129/147 | 153/128 | 0.118 | 1.31 (0.93–1.85) | ||
| >40 | 464/229 | 473/216 | 0.662 | 1.05 (0.84–1.33) | 360/333 | 384/305 | 0.250 | 1.14 (0.91–1.41) | ||
| BMI | 0.793 | 0.319 | ||||||||
| ≤24 | 451/250 | 436/212 | 0.247 | 1.15 (0.91–1.44) | 343/358 | 355/293 | 0.040 | 1.26 (1.01–1.57) | ||
| >24 | 177/91 | 229/93 | 0.283 | 1.22 (0.85–1.75) | 146/122 | 182/140 | 0.868 | 1.03 (0.73–1.44) | ||
| Age at menarche | 0.100 | 0.503 | ||||||||
| ≤15 | 314/182 | 349/149 | 0.019 | 1.38 (1.05–1.82) | 244/252 | 274/224 | 0.074 | 1.26 (0.98–1.63) | ||
| >15 | 314/159 | 316/156 | 0.902 | 0.98 (0.74–1.30) | 245/228 | 263/209 | 0.450 | 1.11 (0.85–1.44) | ||
| Menopausal status | 0.649 | 0.490 | ||||||||
| Premenopausal | 402/218 | 426/198 | 0.261 | 1.15 (0.90–1.46) | 310/310 | 350/274 | 0.056 | 1.25 (0.99–1.57) | ||
| Postmenopausal | 220/122 | 227/100 | 0.162 | 1.27 (0.91–1.78) | 174/168 | 175/152 | 0.605 | 1.09 (0.79–1.49) | ||
| Age at menopause | 0.624 | 0.434 | ||||||||
| ≤50 | 87/56 | 118/49 | 0.471 | 1.19 (0.75–1.89) | 104/95 | 88/79 | 0.862 | 0.96 (0.63–1.48) | ||
| >50 | 133/66 | 109/51 | 0.163 | 1.42 (0.87–2.31) | 70/73 | 87/73 | 0.327 | 1.26 (0.79–2.03) | ||
| Age at first live birth | 0.129 | 0.057 | ||||||||
| ≤25 | 329/195 | 445/198 | 0.017 | 1.36 (1.06–1.75) | 251/273 | 362/281 | 0.010 | 1.37 (1.08–1.74) | ||
| >25 | 270/137 | 191/96 | 0.930 | 0.99 (0.71–1.37) | 216/191 | 149/138 | 0.677 | 0.94 (0.69–1.28) | ||
| ER status | 0.532 | 0.084 | ||||||||
| Positive | 415/229 | 0.057 | 1.23 (0.99–1.53) | 313/331 | 0.009 | 1.32 (1.07–1.61) | ||||
| Negative | 213/112 | 0.480 | 1.10 (0.84–1.45) | 176/149 | 0.931 | 0.99 (0.76–1.28) | ||||
| PR status | 0.923 | 0.148 | ||||||||
| Positive | 369/197 | 0.113 | 1.20 (0.96–1.50) | 275/291 | 0.010 | 1.32 (1.07–1.64) | ||||
| Negative | 259/144 | 0.187 | 1.18 (0.92–1.52) | 214/189 | 0.724 | 1.04 (0.82–1.32) | ||||
aAdjusted by age, BMI, age at menarche, menopausal status and family history of breast cancer where appropriate.
bP for heterogeneity test.
Effects of clinicopathological features and HOTAIR SNPs on breast cancer survival
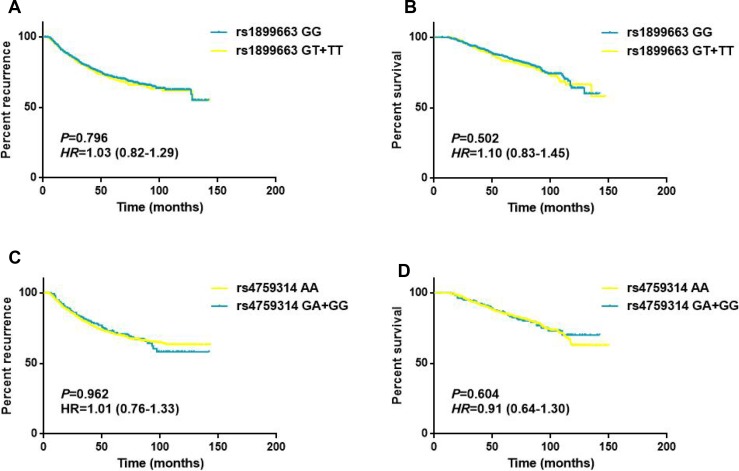
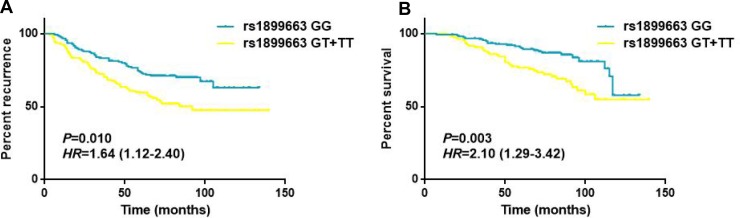
As shown in Table 4, the associations of clinicopathological features and HOTAIR polymorphisms with patients’ disease free survival and overall survival were evaluated by Cox regression analyses. The results demonstrated that tumor size, lymph node involvement and different molecular subtypes were significantly associated with the DFS and OS for breast cancer patients (all P < 0.05, log-rank test). While for the HOTAIR tag SNPs, no statistically significant associations were observed between the genotypes and the survival of breast cancer in any of the genetic models (Figure 2). To further assess the prognostic value of HOTAIR polymorphisms, we also performed stratified analyses by age, tumor size, lymph node involvement and different molecular subtypes. Multivariate analyses revealed that the T carriers of rs1899663 presented significant differences for both DFS (HR = 1.64, 95% CI = 1.12–2.40) and OS (HR = 2.10, 95% CI = 1.29–3.42) in younger patients (age ≤ 40) subgroup (Table 5 and Figure 3). As for the G carriers of rs4759314, we observed a decreased risk for OS (HR = 0.26, 95% CI = 0.08–0.83) in patients without lymph node involvement (Table 6). However, we did not notice any significant difference in survival for rs7958904 (Supplementary Table 2) or within any of the other subgroups of rs1899663 and rs4759314.
Table 4. Multivariate analysis of prognostic factors with DFS and OS for breast cancer patients.
| Characteriscs | Disease free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| Patients (Relapse) | HR (95% CI)a | Log-rank p | Patients (Death) | HR (95% CI)a | Log-rank p | |
| Age | ||||||
| >40 | 693 (229) | 1.00 | 693 (151) | 1.00 | ||
| ≤40 | 276 (108) | 1.17 (0.81–1.67) | 0.404 | 276 (66) | 1.16 (0.74–1.81) | 0.512 |
| Tumor size | ||||||
| ≤2 cm | 385 (81) | 1.00 | 385 (53) | 1.00 | ||
| >2 cm | 584 (256) | 2.44 (1.90–3.14) | <0.01 | 584 (164) | 2.24 (1.65–3.06) | <0.01 |
| Lymph node involvement | ||||||
| No | 479 (104) | 1.00 | 479 (50) | 1.00 | ||
| Yes | 490 (233) | 2.61 (2.07–3.29) | <0.01 | 490 (167) | 3.81 (2.78–5.23) | <0.01 |
| Molecular subtype | ||||||
| Luminal type | 644 (191) | 1.00 | 644 (109) | 1.00 | ||
| HER-2 overexpression | 149 (65) | 1.78 (1.34–2.36) | <0.01 | 149 (46) | 2.16 (1.53–3.05) | <0.01 |
| TNBC | 176 (81) | 1.89 (1.45–2.45) | <0.01 | 176 (62) | 2.56 (1.87–3.49) | <0.01 |
| Adjuvant chemotherapy | ||||||
| Yes | 919 ( 325) | 1.00 | 919 (211) | 1.00 | ||
| No | 50 (12) | 1.46 (0.82–2.61) | 0.201 | 50 (6) | 2.12 (0.94–4.82) | 0.072 |
| rs1899663 G>T | ||||||
| GG | 628 (217) | 1.00 | 628 (137) | 1.00 | ||
| GT | 299 (102) | 0.97 (0.77–1.23) | 0.825 | 299 (69) | 1.06 (0.80–1.42) | 0.685 |
| TT | 42 (18) | 1.53 (0.94–2.48) | 0.086 | 42 (11) | 1.41 (0.76–2.61) | 0.277 |
| GT + TT | 341 (120) | 1.03 (0.82–1.29) | 0.796 | 341 (80) | 1.10 (0.83–1.45) | 0.502 |
| rs4759314 A>G | ||||||
| AA | 801 (275) | 1.00 | 801 (179) | 1.00 | ||
| GA | 157 (61) | 1.06 (0.80–1.40) | 0.669 | 157 (37) | 0.94 (0.66–1.34) | 0.734 |
| GG | 11 (1) | 0.24 (0.03–1.72) | 0.156 | 11 (1) | 0.43 (0.06–3.07) | 0.400 |
| GA + GG | 168 (62) | 1.01 (0.76–1.33) | 0.962 | 168 (38) | 0.91 (0.64–1.30) | 0.604 |
| rs7958904 G>C | ||||||
| GG | 489 (168) | 1.00 | 489 (106) | 1.00 | ||
| GC | 396 (136) | 0.96 (0.76–1.20) | 0.690 | 396 (89) | 0.99 (0.75–1.31) | 0.945 |
| CC | 84 (33) | 1.20 (0.82–1.74) | 0.348 | 84 (22) | 1.25 (0.79–1.98) | 0.339 |
| GC + CC | 480 (169) | 0.99 (0.80–1.23) | 0.959 | 480 (111) | 1.03 (0.79–1.34) | 0.811 |
aData were estimated by Cox regression analyses with adjustment for age, tumor size, lymph node status, ER,PR and Her-2 status where appropriate.
Figure 2. Survival curves for rs1899663 and rs4759314 in total patients.
(A) Disease free survival of the patients grouped according rs1899663 genotypes. (B) Overall survival of the patients grouped according rs1899663 genotypes. (C) Disease free survival of the patients grouped according rs4759314 genotypes. (D) Overall survival of the patients grouped according rs4759314 genotypes.
Table 5. Stratified analysis of HOTAIR rs1899663 genotypes on DFS and OS of breast cancer patients.
| Variable | Disease free survival Genotypes (Relapse/Patients) |
Overall survival Genotypes (Death/Patients) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GG | HR (95% CI) | GT + TT | HR (95% CI)a* | Log-rank p | GG | HR (95% CI) | GT + TT | HR (95% CI)a* | Log-rank p | |
| Age | ||||||||||
| ≤40 | 54/164 | 1.00 | 54/112 | 1.64 (1.12–2.40) | 0.010 | 28/164 | 1.00 | 38/112 | 2.10 (1.29–3.42) | 0.003 |
| >40 | 163/464 | 1.00 | 66/229 | 0.80 (0.60–1.07) | 0.130 | 109/464 | 1.00 | 42/229 | 0.78 (0.54–1.12) | 0.175 |
| Tumor size | ||||||||||
| ≤2 cm | 50/244 | 1.00 | 31/141 | 1.09 (0.70–1.71) | 0.700 | 33/244 | 1.00 | 20/141 | 1.05 (0.60–1.83) | 0.875 |
| >2 cm | 167/384 | 1.00 | 89/200 | 1.05 (0.81–1.36) | 0.719 | 104/384 | 1.00 | 60/200 | 1.14 (0.83–1.57) | 0.407 |
| Lymph node involvement | ||||||||||
| No | 66/322 | 1.00 | 38/157 | 1.21 (0.81–1.80) | 0.356 | 33/322 | 1.00 | 17/157 | 1.09 (0.61–1.95) | 0.779 |
| Yes | 151/306 | 1.00 | 82/184 | 0.87 (0.66–1.14) | 0.304 | 104/306 | 1.00 | 63/184 | 1.04 (0.76–1.43) | 0.807 |
| Molecular subtype | ||||||||||
| Luminal type | 125/415 | 1.00 | 66/229 | 0.95 (0.70–1.28) | 0.727 | 70/415 | 1.00 | 39/229 | 1.04 (0.70–1.54) | 0.861 |
| HER-2 overexpression | 41/98 | 1.00 | 24/51 | 1.24 (0.75–2.05) | 0.411 | 29/98 | 1.00 | 17/51 | 1.22 (0.67–2.22) | 0.512 |
| TNBC | 51/115 | 1.00 | 30/61 | 1.12 (0.71–1.77) | 0.616 | 38/115 | 1.00 | 24/61 | 1.18 (0.70–1.96) | 0.536 |
aCox regression analyses for DFS and OS in breast cancer patients according to dominant model.
*Adjusted by age, tumor size, lymph node status, ER,PR and Her-2 status where appropriate.
Figure 3. Survival curves for rs1899663 in younger patients (age ≤ 40) subgroup.
(A) Disease free survival of the younger subjects (age ≤ 40) grouped according rs1899663 genotypes. (B) Overall survival of the younger subjects (age ≤ 40) grouped according rs1899663 genotypes.
Table 6. Stratified analysis of HOTAIR rs4759314 genotypes on DFS and OS of breast cancer patients.
| Variable | Disease free survival Genotypes (Relapse/Patients) |
Overall survival Genotypes (Death/Patients) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA | HR (95% CI) | GA + GG | HR (95% CI)a* | Log-rank p | AA | HR (95% CI) | GA + GG | HR (95% CI)a* | Log-rank p | |
| Age | ||||||||||
| ≤40 | 85/223 | 1.00 | 23/53 | 1.06 (0.67–1.68) | 0.806 | 52/223 | 1.00 | 14/53 | 1.03 (0.57–1.87) | 0.919 |
| >40 | 190/578 | 1.00 | 39/115 | 0.97 (0.69–1.37) | 0.871 | 127/578 | 1.00 | 24/115 | 0.86 (0.56–1.33) | 0.501 |
| Tumor size | ||||||||||
| ≤2 cm | 65/318 | 1.00 | 16/67 | 1.09 (0.63–1.88) | 0.768 | 46/318 | 1.00 | 7/67 | 0.63 (0.28–1.40) | 0.254 |
| >2 cm | 210/483 | 1.00 | 46/101 | 0.99 (0.71–1.36) | 0.926 | 133/483 | 1.00 | 31/101 | 1.05 (0.71–1.56) | 0.803 |
| Lymph node involvement | ||||||||||
| No | 90/394 | 1.00 | 14/85 | 0.99 (0.97–1.01) | 0.331 | 47/394 | 1.00 | 3/85 | 0.26 (0.08–0.83) | 0.023 |
| Yes | 185/407 | 1.00 | 48/83 | 1.29 (0.94–1.78) | 0.115 | 132/407 | 1.00 | 35/83 | 1.20 (0.83–1.76) | 0.334 |
| Molecular subtype | ||||||||||
| Luminal type | 151/521 | 1.00 | 40/123 | 1.05 (0.74–1.49) | 0.780 | 89/521 | 1.00 | 20/123 | 0.85 (0.52–1.38) | 0.498 |
| HER-2 overexpression | 54/128 | 1.00 | 11/21 | 1.37 (0.71–2.64) | 0.347 | 36/128 | 1.00 | 10/21 | 1.95 (0.96–3.96) | 0.064 |
| TNBC | 70/152 | 1.00 | 11/24 | 0.89 (0.47–1.69) | 0.724 | 54/152 | 1.00 | 8/24 | 0.77 (0.36–1.63) | 0.488 |
aCox regression analyses for DFS and OS in breast cancer patients according to dominant model.
*Adjusted by age, tumor size, lymph node status, ER,PR and Her-2 status where appropriate.
DISCUSSION
Deeper understanding of lncRNAs and their roles in tumor pathogenesis, progression and prognosis could contribute a large number of potential clues to develop novel therapeutic approaches for breast cancer. HOTAIR (HOX transcript antisense RNA) is known as a functional lncRNA which participates in several tumor types including breast cancer [26–40]. The oncogenic roles of HOTAIR have attracted extensive attentions in breast cancer, while epidemiological studies focusing on tumor susceptibility and prognosis conferred by genetic polymorphisms in its locus have not been widely investigated [38–40, 43]. In this present study, we evaluated the effects of three potential functional HOTAIR polymorphisms (rs1899663, rs4759314 and rs7958904) on breast cancer susceptibility and clinical outcomes in a Chinese population. We identified individuals with T allele of rs1899663 and C allele of rs7958904 had an increased risk of developing breast cancer and patients with T carriers of rs1899663 presented a worse DFS and OS in subgroup with younger subjects. Our findings support the hypothesis that the functional genetic variants located in HOTAIR may explain a part of BC genetic basis. And to the best of our knowledge, this is the first study to evaluate the correlations between HOTAIR variants and breast cancer survival.
LncRNA HOTAIR is located on chromosome 12q13.13 and plays a key role in gene regulation by modifying the chromatin structure [41]. The 5′ domain of HOTAIR could bind polycomb repressive complex 2 (PRC2) and leads to a histone H3 lysine27 trimethylation (H3K27me3) in the HOXD locus, while the 3′ domain connects to the LSD1/CoREST/REST complex with H3 lysine 4 demethylation, together regulating the various downstream genes and promoting cancer cell metastasis [42]. HOTAIR has been widely explored in breast cancer and suggested as a functional lncRNA which is correlated with the carcinogenesis, progression and prognosis of BC. The aberrant up-regulation of HOTAIR was proved to be found in breast cancer tissue or plasma samples compared with the normal adjacent tissue or healthy controls [43, 44], and this high expression was also indicated as a predictor of subsequent metastasis and correlated with a shorter survival time of breast cancer patients [38, 43]. Except from these, HOTAIR was additionally reported to be robustly expressed in the basal-like breast cancer cells and the inhibition of HOTAIR could reduce the basal-like gene expression and growth in vitro studies [45]. Therefore, understanding the biological roles of HOTAIR may help us to recruit this lncRNA as a diagnostic or predictive biomarker in breast cancer.
In current study, we demonstrated that the T allelic frequency of rs1899663 and C allelic frequency of rs7958904 were both significantly higher in breast cancer cases compared with the cancer-free controls. Multivariate analyses on genotype distributions also revealed that the TT carriers of rs1899663 and the CC carriers of rs7958904 were consistently associated with the elevated risk of breast cancer. In further stratified analyses, we observed that the T carriers of rs1899663 were correlated with elevated risks of BC in subgroups of younger patients (age ≤ 40), individuals with earlier menarche and subjects with an earlier age at first live birth. As for the C carriers of rs7958904, increased risks of breast cancer were found to be more evident in subgroup of lower BMI individuals (BMI ≤ 24), individuals with an earlier age at first live birth and patients with ER positive or PR positive. These results showed that the effects of HOTAIR genetic variant on breast cancer risk could be modulated by specific environmental exposures as well as demographic factors, and provided evidence supporting that the carcinogenesis is a complex process involving both genetic and environmental factors. Previous studies have suggested that the T allele of rs1899663 was associated with a higher risk of developing prostate cancer [48], whereas this significant positive correlation was not detected in cervical cancer [49] and esophageal squamous cell carcinoma [50]. In one study concerning HOTAIR polymorphisms and breast cancer [51], the rs1899663 T allele also did not show significant differences in the frequency distribution of cancer patients and healthy controls in an overall correlation analysis, while the follow-up stratified analysis indicated the GT+TT genotypes had a significantly lower risk of BC among women with age at menarche >14 (OR = 0.42, 95% CI = 0.21–0.82) and number of pregnancies >2 (OR = 0.65, 95% CI = 0.49–0.95). As for rs7958904, several studies have indicated that the C allele was associated with a significantly decreased risk of colorectal cancer [52], ovarian cancer [53] and osteosarcoma [54] when compared with the G allele, which produce a contrary result with our study. This may be interpreted by the different susceptibilities to a disease among the different populations and the different kinds of cancer could have various etiologies, which involve diverse genetic or epigenetic modifications.
The polymorphism rs1899663 and rs7958904 was separately located on the intron 2 and exon 6 of HOTAIR gene. Guo et al. [55] have reported that HOTAIR SNP rs12826786 which is in strong LD with rs1899663 (r2 = 0.983) was associated with gastric cardia adenocarcinoma risk and had an allelic-specific effect on HOTAIR expression. It is plausible that the rs1899663 or its LD polymorphisms could affect the BC susceptibility by altering the HOTAIR expressions. In silico analyses have revealed that, the secondary structure of HOTAIR gene was distinctly changed with the rs7958904 G/C variants, indicating that this polymorphism may participate in tumorigenesis through the alteration of HOTAIR structure [52]. Another explanation for rs7958904 in relation to breast cancer susceptibility is that the real functional SNP is rs920778, which is in high LD (r2 = 0.984) with rs7958904. Polymorphism rs920788 was also located on the intron of HOTAIR gene and was proved to be able to enhance the intronic enhancer activity and increase HOTAIR expression in several cancer cells [49, 50].
In overall survival study, we did not notice any significant association between genotypes of three tag SNPs and the survival of breast cancer in any of the genetic models. While in the subsequent stratified analysis, we revealed that the T allele of rs1899663 presented significant differences for both DFS (HR = 1.64, 95% CI = 1.12–2.40) and OS (HR = 2.10, 95% CI = 1.29–3.42) in younger subjects (age ≤ 40) and the G allele of rs4759314 showed a decreased risk for OS (HR = 0.26, 95% CI = 0.08–0.83) in patients without lymph node involvement. However, given the small sample of rs4759314 GG carriers in subgroups without lymph node involvement in overall analysis (3 cases), we speculated that the association of rs4759314 observed in OS study may be a false positive result.
In conclusion, we identified two SNPs located in HOTAIR (rs1899663 and rs7958904) that were significantly associated with the increased risk of breast cancer and firstly investigated the role of HOTAIR tag SNPs on the clinical outcome of BC in a southeast Chinese Han population. However, several limitations in this study should also be mentioned. Firstly, the sample size of the current study was still not large enough and might lead to a limited statistical power and impact on the accuracy and precision of the results. Secondly, we only included three lncRNA HOTAIR polymorphisms in the present study, while studies comprising more functional SNPs in HOTAIR might be more able to illuminate the precise role of genetic variants in BC carcinogenesis and progress. Thirdly, the biological function of the HOTAIR polymorphisms is not clear, further functional studies are still needed to explore the relationship. In spite of these limitations, the findings of our study were still informative for the researchers and physicians in this field. Additional prospective population-based studies with larger sample size and different ethnicities, as well as relevant functional studies are still needed to confirm our findings.
MATERIALS AND METHODS
Ethical statement
This study and consent procedure was approved by the Ethical Committee of Affiliated Union Hospital of Fujian Medical University. Each participant included in the study has provided a written informed consent document.
Study subjects
This hospital-based study was conducted on a total of 969 breast cancer patients and 970 healthy free controls. All participants were genetically unrelated Chinese Han residents of Fujian Province and its surrounding regions. Breast cancer subjects were all histopathologically confirmed with primary breast cancer and recruited from the Affiliated Union Hospital of Fujian Medical University between July 1995 and October 2010. Healthy controls (frequency-matched to cases on age ±3 years) were randomly selected from individuals attending routine health examination in the outpatients’ department during the same period. Each patient and healthy control was interviewed face-to-face by two trained oncologists to gather information on demographic factors, menstrual status, fertility status, previous benign breast disease history and the family history of breast cancer. Specific clinicopathological data of breast cancer cases including tumor size, lymph node involvement, estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER-2) status were all extracted from medical records and pathology reports. The molecular subtypes of breast cancer based on immunohistochemical (IHC) profiles were categorized as follows: Luminal subtype = ER+ or PR+, and HER2±; HER2 overexpression (HER2+) = ER−, PR−, and HER2+; Triple-negative breast cancer (TNBC) = ER−, PR−, and HER2−.
Outcome collections
Disease free survival (DFS) and overall survival (OS) were the main study points. Patients alive on the last follow-up date were considered censored. DFS was measured as the time from the date of diagnosis to the first local or distant recurrence or to the last follow-up. OS was defined as the time from the date of diagnosis to the date of death due to all causes (including breast cancer) or the last follow-up. The date of death was obtained from inpatient and outpatient records or by the relatives of patients through follow-up telephone calls. The last follow-up date of this study was November 1st, 2016.
DNA extraction and genotyping
Each participant was asked to provide a 5-ml peripheral blood sample after enrolling in this study. Genomic DNA was extracted from the peripheral-blood samples using a Whole-Blood DNA Extraction Kit (Bioteke, Beijing, China) following the manufacturer’s instructions. LncRNA HOTAIR tag SNPs were genotyped by a 2 × 48-Plex SNPscan Kit (Cat#:G0104K; Genesky Biotechnologies Inc., Shanghai, China). The DNA samples were ligated and amplified by polymerase chain reaction (PCR) according to the standardization protocol recommended by the manufacturer. Ligation products were performed with an ABI3730XL sequencer and the raw data was analyzed by GeneMapper 4.1 Software (Applied Biosystems, Foster City, CA). For quality control, all genotyping were performed without knowledge of case or control status. About 10% of the DNA samples were randomly selected for direct sequencing (BGI Sequencing, Beijing), and the result was 100% concordant.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, version 21.0) for Windows (SPSS, Chicago, IL). The differences between breast cancer cases and healthy controls in demographic characteristics and environmental risk factors were evaluated by using the Student’s t-test (for continuous variables) and chi-squared (χ2) test (for categorical variables). Hardy-Weinberg equilibrium (HWE) was applied by a goodness-of-fit chi-squared (χ2) test to assess the expected and observed genotype frequencies in control subjects. Associations among genotypes, breast cancer risk and survival were evaluated by the computing odds ratio (OR), hazard ratio (HR) and its 95% confidence interval (CI) from univariate and multivariate logistic regression analyses. Linkage disequilibrium was calculated from genotype data using Haploview 4.1 (http://www.broad.mit.edu/mpg/haploview/). The power analysis of this study was performed by using the QUANTO program, version 1.2.4, with the disease risk for the Chinese population was 268 per 100000. The disease free survival and overall survival was calculated by the Kaplan–Meier method, with the log-rank test used to compare the differences. All statistical analyses were two-sided, and a level of P value less than 0.05 was considered significant.
SUPPLEMENTARY MATERIALS TABLES
Footnotes
Author contributions
WC and GWH conceived and designed the experiments. LSP and LN performed the experiments. FFM analyzed the data. LYX wrote the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
The authors have declared that no competing interests exist.
FUNDING
The study was supported by grants from National Nature Science Foundation (No.81302320), Foundation for Young Talent Training Program of Fujian Provincial Health and Family Planning Commission (2015-ZQN-ZD-14) and Sci-Tech Key Program of Fujian Province (2015J01473, 2016J01549).
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. https://doi.org/10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. https://doi.org/10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE. Breast cancer in China. Lancet Oncol. 2014;15:e279–89. doi: 10.1016/S1470-2045(13)70567-9. https://doi.org/10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. https://doi.org/10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–40. doi: 10.1016/S1470-2045(00)00254-0. https://doi.org/10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 6.Trentham-Dietz A, Sprague BL, Hampton JM, Miglioretti DL, Nelson HD, Titus LJ, Egan KM, Remington PL, Newcomb PA. Modification of breast cancer risk according to age and menopausal status: a combined analysis of five population-based case-control studies. Breast Cancer Res Treat. 2014;145:165–75. doi: 10.1007/s10549-014-2905-y. https://doi.org/10.1007/s10549-014-2905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter CL, Ross RK, Paganini-Hill A, Bernstein L. Effect of family history, obesity and exercise on breast cancer risk among postmenopausal women. Int J Cancer. 2003;106:96–102. doi: 10.1002/ijc.11186. https://doi.org/10.1002/ijc.11186. [DOI] [PubMed] [Google Scholar]
- 8.Lacey JV, Jr, Kreimer AR, Buys SS, Marcus PM, Chang SC, Leitzmann MF, Hoover RN, Prorok PC, Berg CD, Hartge P, Prostate LC, Ovarian Cancer Screening Trial Project Team Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer. 2009;9:84. doi: 10.1186/1471-2407-9-84. https://doi.org/10.1186/1471-2407-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb PM, Byrne C, Schnitt SJ, Connolly JL, Jacobs T, Peiro G, Willett W, Colditz GA. Family history of breast cancer, age and benign breast disease. Int J Cancer. 2002;100:375–8. doi: 10.1002/ijc.10490. https://doi.org/10.1002/ijc.10490. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, Suman VJ, Johnson J, Blake C, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–37. doi: 10.1056/NEJMoa044383. https://doi.org/10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 11.Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A, Wang J, Yu K, Chatterjee N, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–4. doi: 10.1038/ng2075. https://doi.org/10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudet MM, Kirchhoff T, Green T, Vijai J, Korn JM, Guiducci C, Segre AV, McGee K, McGuffog L, Kartsonaki C, Morrison J, Healey S, Sinilnikova OM, et al. Common genetic variants and modification of penetrance of BRCA2-associated breast cancer. PLoS Genet. 2010;6:e1001183. doi: 10.1371/journal.pgen.1001183. https://doi.org/10.1371/journal.pgen.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniou AC, Kartsonaki C, Sinilnikova OM, Soucy P, McGuffog L, Healey S, Lee A, Peterlongo P, Manoukian S, Peissel B, Zaffaroni D, Cattaneo E, Barile M, et al. Common alleles at 6q25.1 and 1p11.2 are associated with breast cancer risk for BRCA1 and BRCA2 mutation carriers. Hum Mol Genet. 2011;20:3304–21. doi: 10.1093/hmg/ddr226. https://doi.org/10.1093/hmg/ddr226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Q, Zhang B, Sung H, Low SK, Kweon SS, Lu W, Shi J, Long J, Wen W, Choi JY, Noh DY, Shen CY, Matsuo K, et al. Genome-wide association analysis in East Asians identifies breast cancer susceptibility loci at 1q32.1, 5q14.3 and 15q26.1. Nat Genet. 2014;46:886–90. doi: 10.1038/ng.3041. https://doi.org/10.1038/ng.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong Q, Yu S, Yang Y, Liu G, Shao Z. A polymorphism in JMJD2C alters the cleavage by caspase-3 and the prognosis of human breast cancer. Oncotarget. 2014;5:4779–87. doi: 10.18632/oncotarget.2029. https://doi.org/10.18632/oncotarget.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr N, Dudbridge F, Dryden N, Maguire S, Novo D, Perrakis E, Johnson N, Ghoussaini M, Hopper JL, Southey MC, Apicella C, Stone J, Schmidt MK, et al. Fine-mapping identifies two additional breast cancer susceptibility loci at 9q31.2. Hum Mol Genet. 2015;24:2966–84. doi: 10.1093/hmg/ddv035. https://doi.org/10.1093/hmg/ddv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Fu F, Lin Y, Qiu L, Lu M, Zhang J, Qiu W, Yang P, Wu N, Huang M, Wang C. The precision relationships between eight GWAS-identified genetic variants and breast cancer in a Chinese population. Oncotarget. 2016;7:75457–67. doi: 10.18632/oncotarget.12255. https://doi.org/10.18632/oncotarget.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41. doi: 10.1016/j.cell.2009.02.006. https://doi.org/10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Rinn JL. lncRNAs: linking RNA to chromatin. Cold Spring Harb Perspect Biol. 2014:6. doi: 10.1101/cshperspect.a018614. https://doi.org/10.1101/cshperspect.a018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, Manos PD, Datta S, Lander ES, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42:1113–7. doi: 10.1038/ng.710. https://doi.org/10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchese FP, Huarte M. Long non-coding RNAs and chromatin modifiers: their place in the epigenetic code. Epigenetics. 2014;9:21–6. doi: 10.4161/epi.27472. https://doi.org/10.4161/epi.27472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morlando M, Ballarino M, Fatica A, Bozzoni I. The role of long noncoding RNAs in the epigenetic control of gene expression. ChemMedChem. 2014;9:505–10. doi: 10.1002/cmdc.201300569. https://doi.org/10.1002/cmdc.201300569. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Guo Q, Chen J, Hu J, Wang S, Sun Y. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigation. Oncol Rep. 2014;31:358–64. doi: 10.3892/or.2013.2850. https://doi.org/10.3892/or.2013.2850. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Liu Y, Zhang W, Zhou Z, Wu J, Cui P, Zhang Y, Huang G. Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle. 2015;14:3112–23. doi: 10.1080/15384101.2015.1078034. https://doi.org/10.1080/15384101.2015.1078034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P, Zhang G, Li J, Yang R, Chen S, Wu S, Zhang F, Bai Y, Zhao H, Wang Y, Dun S, Chen X, Sun Q, et al. Long Noncoding RNA RGMB-AS1 Indicates a Poor Prognosis and Modulates Cell Proliferation, Migration and Invasion in Lung Adenocarcinoma. PLoS One. 2016;11:e0150790. doi: 10.1371/journal.pone.0150790. https://doi.org/10.1371/journal.pone.0150790. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Ishibashi M, Kogo R, Shibata K, Sawada G, Takahashi Y, Kurashige J, Akiyoshi S, Sasaki S, Iwaya T, Sudo T, Sugimachi K, Mimori K, Wakabayashi G, et al. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 2013;29:946–50. doi: 10.3892/or.2012.2219. https://doi.org/10.3892/or.2012.2219. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Zhou L, Wu LM, Lai MC, Xie HY, Zhang F, Zheng SS. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243–50. doi: 10.1245/s10434-011-1581-y. https://doi.org/10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Wu Z, Mei Q, Li X, Guo M, Fu X, Han W. Long non-coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br J Cancer. 2013;109:2266–78. doi: 10.1038/bjc.2013.548. https://doi.org/10.1038/bjc.2013.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen FJ, Sun M, Li SQ, Wu QQ, Ji L, Liu ZL, Zhou GZ, Cao G, Jin L, Xie HW, Wang CM, Lv J, De W, et al. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol Carcinog. 2013;52:908–15. doi: 10.1002/mc.21944. https://doi.org/10.1002/mc.21944. [DOI] [PubMed] [Google Scholar]
- 30.Lv XB, Lian GY, Wang HR, Song E, Yao H, Wang MH. Long noncoding RNA HOTAIR is a prognostic marker for esophageal squamous cell carcinoma progression and survival. PLoS One. 2013;8:e63516. doi: 10.1371/journal.pone.0063516. https://doi.org/10.1371/journal.pone.0063516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa T, Endo H, Yokoyama M, Abe J, Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T, Satoh K. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun. 2013;436:319–24. doi: 10.1016/j.bbrc.2013.05.101. https://doi.org/10.1016/j.bbrc.2013.05.101. [DOI] [PubMed] [Google Scholar]
- 32.Liu XH, Liu ZL, Sun M, Liu J, Wang ZX, De W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13:464. doi: 10.1186/1471-2407-13-464. https://doi.org/10.1186/1471-2407-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ono H, Motoi N, Nagano H, Miyauchi E, Ushijima M, Matsuura M, Okumura S, Nishio M, Hirose T, Inase N, Ishikawa Y. Long noncoding RNA HOTAIR is relevant to cellular proliferation, invasiveness, and clinical relapse in small-cell lung cancer. Cancer Med. 2014;3:632–42. doi: 10.1002/cam4.220. https://doi.org/10.1002/cam4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, Yu PF, Cheng XD. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587–97. doi: 10.7150/ijbs.6339. https://doi.org/10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, Sato I, Yamaguchi K, Tanaka N, Iijima K, Shimosegawa T, Sugamura K, et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8:e77070. doi: 10.1371/journal.pone.0077070. https://doi.org/10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emadi-Andani E, Nikpour P, Emadi-Baygi M, Bidmeshkipour A. Association of HOTAIR expression in gastric carcinoma with invasion and distant metastasis. Adv Biomed Res. 2014;3:135. doi: 10.4103/2277-9175.133278. https://doi.org/10.4103/2277-9175.133278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, Goel A. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35:2731–9. doi: 10.1093/carcin/bgu200. https://doi.org/10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6. doi: 10.1038/nature08975. https://doi.org/10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu L, Zhu G, Zhang C, Deng Q, Katsaros D, Mayne ST, Risch HA, Mu L, Canuto EM, Gregori G, Benedetto C, Yu H. Association of large noncoding RNA HOTAIR expression and its downstream intergenic CpG island methylation with survival in breast cancer. Breast Cancer Res Treat. 2012;136:875–83. doi: 10.1007/s10549-012-2314-z. https://doi.org/10.1007/s10549-012-2314-z. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen KP, Thomassen M, Tan Q, Bak M, Cold S, Burton M, Larsen MJ, Kruse TA. Long non-coding RNA HOTAIR is an independent prognostic marker of metastasis in estrogen receptor-positive primary breast cancer. Breast Cancer Res Treat. 2013;142:529–36. doi: 10.1007/s10549-013-2776-7. https://doi.org/10.1007/s10549-013-2776-7. [DOI] [PubMed] [Google Scholar]
- 41.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–23. doi: 10.1016/j.cell.2007.05.022. https://doi.org/10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93. doi: 10.1126/science.1192002. https://doi.org/10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorensen KP, Thomassen M, Tan Q, Bak M, Cold S, Burton M, Larsen MJ, Kruse TA. Long non-coding RNA HOTAIR is an independent prognostic marker of metastasis in estrogen receptor-positive primary breast cancer. Breast Cancer Res Treat. 2013;142:529–36. doi: 10.1007/s10549-013-2776-7. https://doi.org/10.1007/s10549-013-2776-7. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Zhang K, Luo Z, Liu L, Wu L, Liu J. Circulating long non-coding HOX transcript antisense intergenic ribonucleic acid in plasma as a potential biomarker for diagnosis of breast cancer. Thorac Cancer. 2016;7:627–32. doi: 10.1111/1759-7714.12373. https://doi.org/10.1111/1759-7714.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuang Y, Nguyen HT, Burow ME, Zhuo Y, El-Dahr SS, Yao X, Cao S, Flemington EK, Nephew KP, Fang F, Collins-Burow B, Rhodes LV, Yu Q, et al. Elevated expression of long intergenic non-coding RNA HOTAIR in a basal-like variant of MCF-7 breast cancer cells. Mol Carcinog. 2015;54:1656–67. doi: 10.1002/mc.22237. https://doi.org/10.1002/mc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan R, Cao J, Song C, Chen Y, Wu Z, Wang K, Dai L. Polymorphisms in lncRNA HOTAIR and susceptibility to breast cancer in a Chinese population. Cancer Epidemiol. 2015;39:978–85. doi: 10.1016/j.canep.2015.10.025. https://doi.org/10.1016/j.canep.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 47.Bayram S, Sumbul AT, Dadas E. A functional HOTAIR rs12826786 C>T polymorphism is associated with breast cancer susceptibility and poor clinicopathological characteristics in a Turkish population: a hospital-based case-control study. Tumour Biol. 2016;37:5577–84. doi: 10.1007/s13277-015-4430-y. https://doi.org/10.1007/s13277-015-4430-y. [DOI] [PubMed] [Google Scholar]
- 48.Taheri M, Habibi M, Noroozi R, Rakhshan A, Sarrafzadeh S, Sayad A, Omrani MD, Ghafouri-Fard S. HOTAIR genetic variants are associated with prostate cancer and benign prostate hyperplasia in an Iranian population. Gene. 2017;613:20–4. doi: 10.1016/j.gene.2017.02.031. https://doi.org/10.1016/j.gene.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 49.Guo L, Lu X, Zheng L, Liu X, Hu M. Association of Long Non-Coding RNA HOTAIR Polymorphisms with Cervical Cancer Risk in a Chinese Population. PLoS One. 2016;11:e0160039. doi: 10.1371/journal.pone.0160039. https://doi.org/10.1371/journal.pone.0160039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Zhou L, Fu G, Sun F, Shi J, Wei J, Lu C, Zhou C, Yuan Q, Yang M. The identification of an ESCC susceptibility SNP rs920778 that regulates the expression of lncRNA HOTAIR via a novel intronic enhancer. Carcinogenesis. 2014;35:2062–7. doi: 10.1093/carcin/bgu103. https://doi.org/10.1093/carcin/bgu103. [DOI] [PubMed] [Google Scholar]
- 51.Yan R, Cao J, Song C, Chen Y, Wu Z, Wang K, Dai L. Polymorphisms in lncRNA HOTAIR and susceptibility to breast cancer in a Chinese population. Cancer Epidemiol. 2015;39:978–85. doi: 10.1016/j.canep.2015.10.025. https://doi.org/10.1016/j.canep.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 52.Xue Y, Gu D, Ma G, Zhu L, Hua Q, Chu H, Tong N, Chen J, Zhang Z, Wang M. Genetic variants in lncRNA HOTAIR are associated with risk of colorectal cancer. Mutagenesis. 2015;30:303–10. doi: 10.1093/mutage/geu076. https://doi.org/10.1093/mutage/geu076. [DOI] [PubMed] [Google Scholar]
- 53.Wu H, Shang X, Shi Y, Yang Z, Zhao J, Yang M, Li Y, Xu S. Genetic variants of lncRNA HOTAIR and risk of epithelial ovarian cancer among Chinese women. Oncotarget. 2016;7:41047–52. doi: 10.18632/oncotarget.8535. https://doi.org/10.18632/oncotarget.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Q, Chen F, Fei Z, Zhao J, Liang Y, Pan W, Liu X, Zheng D. Genetic variants of lncRNA HOTAIR contribute to the risk of osteosarcoma. Oncotarget. 2016;7:19928–34. doi: 10.18632/oncotarget.7957. https://doi.org/10.18632/oncotarget.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo W, Dong Z, Bai Y, Guo Y, Shen S, Kuang G, Xu J. Associations between polymorphisms of HOTAIR and risk of gastric cardia adenocarcinoma in a population of north China. Tumour Biol. 2015;36:2845–54. doi: 10.1007/s13277-014-2912-y. https://doi.org/10.1007/s13277-014-2912-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.