Figure 2. PARP-1 is overexpressed in patients with advanced MF and Sezary syndrome.
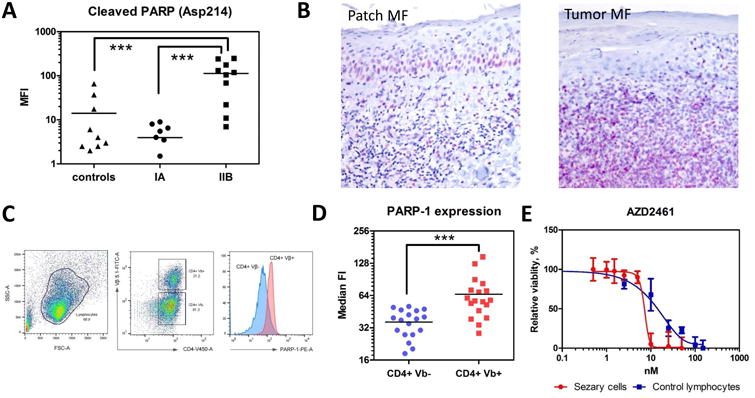
(A) Cleaved PARP (Asp214) by Luminex in the peripheral blood of patients with MF: stage IA (n=7), stage IIB (tumors) (n=10) and healthy volunteers (controls) (n=9). ***, p < 0.001. (B) A representative sample of PARP-1 expression in a patient with patch stage MF (patch MF) vs. a patient with tumor stage MF (tumor MF). IHC, × 200. (C) The gating strategy to determine the PARP-1 expression on CD4+Vβ+ (malignant) and CD4+Vβ- (non-malignant) lymphocytes. (D) The median fluorescent intensity (FI) of intracellular PARP-1 in CD4+Vβ+ (malignant) and CD4+Vβ- (non-malignant) lymphocytes in patients with Sézary syndrome (n=18). ***, p < 0.001. (E) Effect of AZD2461 on the viability of Sezary cells. Cell viability by XTT colorimetric assay 24 hr after co-incubation of lymphocytes with different PARPi. Sézary cells (red) vs. control lymphocytes (blue). AZD2461 doses are shown in the X-axis, whereas the Y-axis shows the relative cell viability.
