Abstract
Electromembrane extraction (EME) is an analytical microextraction technique, where charged analytes (such as drug substances) are extracted from an aqueous sample (such as a biological fluid), through a supported liquid membrane (SLM) comprising a water immiscible organic solvent, and into an aqueous acceptor solution. The driving force for the extraction is an electrical potential (dc) applied across the SLM. In this paper, EME is reviewed. First, the principle for EME is explained with focus on extraction of cationic and anionic analytes, and typical performance data are presented. Second, papers published in 2016 are reviewed and discussed with focus on (a) new SLMs, (b) new support materials for the SLM, (c) new sample additives improving extraction, (d) new technical configurations, (e) improved theoretical understanding, and (f) pharmaceutical new applications. Finally, important future research objectives and directions are defined for further development of EME, with the aim of establishing EME in the toolbox of future analytical laboratories.
Keywords: Sample preparation, Microextraction, Electromembrane extraction
1. Introduction
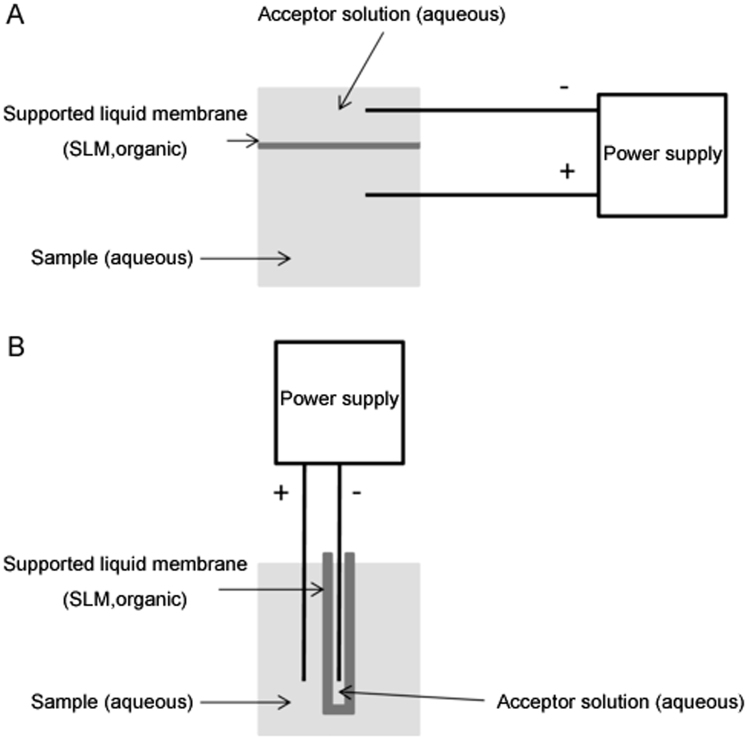
Electromembrane extraction (EME) is a liquid-phase microextraction concept intended for selective extraction and pre-concentration of target analytes from aqueous samples. Target analytes are typically drug substances, and aqueous samples are typically whole blood and urine. Thus, EME is well suited for pharmaceutical analysis, but environmental and food applications are also frequently seen. EME was published for the first time in 2006 [1]. EME was motivated and developed from hollow-fibre liquid-phase microextraction (LPME) [2], which in turn was developed based on the idea of solid-phase microextraction (SPME) [3], single-drop microextraction (SDME) [4], and supported liquid membrane extraction [5]. The principle of EME is illustrated in Fig. 1. Target analytes are extracted from the sample, through a supported liquid membrane (SLM), and into an acceptor solution. This mass transfer is by electrokinetic migration facilitated by an external electrical field sustained across the SLM. The SLM comprises an organic solvent (5–25 μL) immobilized by capillary forces in the pores of a porous polymeric membrane. This porous polymeric support can be a flat sheet or a hollow fibre membrane as illustrated in Fig. 1.
Fig. 1.
Principle of EME. (A) SLM immobilized in flat sheet porous polymeric membrane. (B) SLM immobilized in hollow fibre porous polymeric membrane.
For extraction of cationic analytes (basic substances), pH in the sample and in the acceptor solution is neutral or acidic to maintain the analytes in their protonated state. Thus, as positively charged species, the target analytes are prone to electrokinetic migration. The cathode is located in the acceptor solution and the anode is located in the sample. For extraction of anionic analytes, the direction of the electrical field is reversed, and pH in the sample and acceptor solution is neutral or alkaline to maintain the analytes negatively charged. Typical experimental conditions and performance data for EME are illustrated in Table 1 with methadone (non-polar basic drug), diclofenac (non-polar acidic drug), and buserelin (polar peptide) as examples [6], [7], [8]. The extractions were from plasma and urine, and with typical SLM compositions. For EME of the cationic substances, methadone and buserelin, the cathode was located in the acceptor solution. For extraction of diclofenac (anionic substance), the polarity was reversed. The pH in sample and acceptor solutions was selected to ensure full ionization of the analytes. For EME of buserelin, a hydrophobic ion-pair reagent (negative charge) was added to the SLM to facilitate transfer of the polar peptide (positive charge) into the SLM.
Table 1.
Typical experimental conditions and performance data for methadone (non-polar basic drug) [6], diclofenac (non-polar acidic drug) [7], and buserelin (polar peptide) [8].
| Experimental conditions and performance data | Drugs |
||
|---|---|---|---|
| Methadone (non-polar basic drug) | Diclofenac (non-polar acidic drug) | Buserelin (polar peptide) | |
| Experimental conditions | |||
| Sample | 600 μL plasma (pH 7.4) | 7 mL urine (pH 12.0) | 625 μL plasma+4375 μL water |
| SLM | 10 μL 2-nitrophenyl octyl ether (NPOE) | 1-Octanol | 95% of 1-octanol and 5% di-(2-ethylhexyl)- phosphate |
| Acceptor solution | 600 μL 20 mM formic acid | 20 μL 50 mM NaOH | 30 μL aqueous solution (pH 1) |
| Voltage | 300 V | 40 V | 20 V |
| Polarity | Cathod (-) in acceptor | Anode (+) in acceptor | Cathode (-) in acceptor |
| Extraction time | 30 min | 15 min | 15 min |
| Performance data | |||
| Recovery | 30% | 89% | 58% |
| Relative standard deviation | 9% (n=6, 100 ng/mL) | 7% | 9% (100 ng/mL) |
The scientific interest for EME is related to several unique conceptual properties. First, extraction selectivity can be manipulated by the direction and magnitude of the electrical field [9]. The direction of the electrical field is controlled by the external power supply, and is used to tune the extraction system for either cationic or anionic analytes. The magnitude of the electrical field is also controlled by the external power supply, and research has demonstrated that extraction selectivity is dependent on the magnitude of the electrical field [10]. Second, extraction selectivity is controlled by the chemical composition of the SLM. The SLM can be tuned for non-polar analytes [11], polar analytes [11], or for highly selective extraction of certain analytes based on molecular recognition and complexation [12]. Third, extraction selectivity is controlled by the pH conditions in the sample and acceptor solution [13].
Additional advantages of EME include efficient sample clean-up [14], [15]. Due to the non-polar nature of the SLM, many matrix components present in aqueous samples such as biological fluids are unable to pass the SLM, and they remain in the sample. In addition, anionic species will remain in the sample during extraction of cationic analytes, and vice versa, due to the direction of the electrical filed. Acceptor solutions in EME are (in most cases) aqueous; therefore, they can be injected directly into liquid chromatography (HPLC), liquid chromatography–mass spectrometry (LC–MS), and capillary electrophoresis (CE) [16]. Thus, there is no need for evaporation of extracts and reconstitution, as is often the case with traditional sample preparation methods. Normally, the volume of sample in EME exceeds the volume of acceptor solution; therefore analytes can be pre-concentrated during the process. In one example, EME from 3.5 mL samples and into 20 μL acceptor solution resulted in 74 times the target analyte pre-concentration [17]. Finally, the volume of organic solvent used for the SLM is in the range of 3–15 μL, and this represents the total volume of organic solvent required per sample. Thus, EME represents a green chemistry approach to analytical sample preparation.
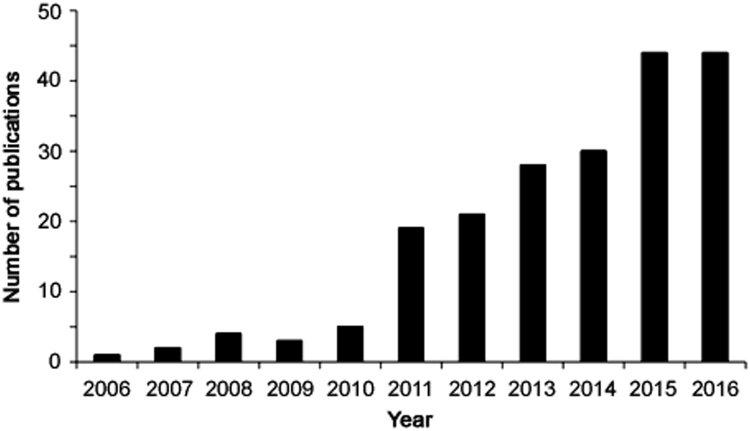
Since the introduction in 2006, nearly 250 research papers have been published on EME, and in the last couple of years nearly 50 research papers have been published per year (Fig. 2). The publication frequency is positive and EME is an active research area. EME has been reviewed several times in recent years [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. The literature comprises research articles on applications of EME, technical developments, and fundamental issues. Applications reported include a broad range of chemical targets, but the use of EME for extraction of drug substances from biological fluids (blood, urine, and saliva) and inorganic ions from environmental waters dominates most recently. The purpose of the current paper is to review the most recent literature to give a flavour of the most recent development and to address one very important question: what are the future directions and perspectives of EME? Thus, in the subsequent section, selected original research papers published in 2016 are discussed and put into context.
Fig. 2.
Number of EME publications per year from 2006 and up to date (Scopus).
2. Recent trends
The EME research published in 2016 falls within the following categories: (a) new SLMs, (b) new support materials for the SLM, (c) new sample additives improving mass transfer, (d) pharmaceutical new technical configurations, (e) improved theoretical understanding, and (f) new applications. This pattern is characteristic also for EME papers published before 2016.
2.1. New SLMs
Since the introduction in 2006, most EME has been conducted with 2-nitrophenyl octyl ether (NPOE) as the SLM. For non-polar basic analytes (log P>2), NPOE is an ideal solvent. Thus, NPOE fulfils the following criteria: (1) provides high recovery for target analyte, (2) provides a high level of sample clean-up, (3) provides low extraction current, (4) does not leak into the sample or acceptor solution, and (5) does not contain solvent impurities which can contaminate the acceptor solution. Criteria (1) and (2) are general requirements for successful sample preparation. On the other hand, criterion (3) is EME specific, because an electrical field is coupled across the SLM, and the current is flowing in the system (extraction current). The extraction current is due to transfer of (a) analyte ions and (b) background ions across the SLM, and causes electrolysis at the electrodes in the sample and acceptor solution. With high extraction current, bubble formation may occur along the electrodes, and pH conditions may change in case of insufficient buffer capacity. Thus, the extraction current should be kept as low as possible (<50 μA) and preferably it should be less than 10 μA. Criterion (4) is related to the stability and integrity of the SLM. The SLM solvent should be hydrophobic to avoid leakage into the aqueous sample and acceptor solution. Criterion (5) is challenging, and based on testing of many different organic solvents, we have experienced that solvents often fail due to leakage of solvent impurities into the acceptor solution. Thus, even after EME from pure laboratory water, several background peaks may be observed in the final chromatogram using solvents non-compliant with criterion (5).
For non-polar basic analytes, a limited number of alternative solvents have been used, including 2-nitrophenyl pentyl ether [13], 1-ethyl-2-nitrobenzene [32], 1-isopropyl-4-nitrobenzene [33], 2-octanone [34], and 2-nonanone [31]. These are all general SLMs for non-polar basic analytes, providing a similar type of selectivity. Generally, the successful EME solvents for non-polar basic analytes have very high hydrogen bond acceptor basicity, zero hydrogen bond donor acidity, and 3< log P<5.5 [35]. These properties are important because the protonated basic analytes are transferred and dissolved into the SLM mainly by hydrogen bond interactions. To some extent, mass transfer for non-polar basic analytes can, therefore, be predicted. For polar basic analytes (log P<2), the use of pure solvents has been unsuccessful until recently, and hydrophobic ion-pair reagents such as di(2-ethylhexyl) phosphate (DEHP) have been added to the SLM (typically NPOE) to facilitate mass transfer [11]. This, however, also increases the extraction current, and EME systems based on NPOE mixed with DEHP are less stable. For acidic analytes, aliphatic alcohols have been the most successful SLMs [31], but these are less stable than NPOE in contact with biological fluids.
For further development of EME there is a need for discovery of new SLMs, especially for polar basic analytes and acidic analytes. In addition, development of more selective or specific SLMs will be of great interest for future applications. High specificity in the extraction step may reduce the costs and complexity of the final detection system. New SLMs have been addressed recently in several research papers, both using pure solvents [30], [34], [35] and solid additives in the SLM [12], [36], [37], [38]. With respect to pure solvents, a range of alkylated phosphates and phosphites have been studied recently, and tributyl phosphate and bis(2-ethylhexyl) phosphite (DEHPi) were identified as new solvents providing efficient extraction of polar basic analytes [35], [39]. This represented an important step forward, and for the first time polar basic analytes were extracted without an ion-pair reagent in the SLM. The new SLMs are non-ionic (containing no ion-pair reagent) and therefore, provided low extraction current. Recently, a review has been published solely focusing on the accumulated experiences with different organic solvents in EME [31].
In addition to pure solvents, different SLM additives have been proposed recently to enhance mass transfer [12], [36], [38], [40], [41]. In one very interesting paper, the non-ionic macrocyclic compound bambus [6] uril was added to nitrobenzene and used as SLM for extraction of inorganic anions [12]. Bambus [6] uril provided strong host-guest interactions with selected inorganic anions. Thus, with this new SLM, iodide, bromide, and perchlorate were extracted with high efficiency, whereas major anions such as chloride, nitrate, sulphate, and carbonate were totally discriminated. The specificity of this EME system was remarkable, and addition of such species-specific macrocyclic modifiers should definitely be intensified in the near future. Another approach to enhancing selectivity has been reported very recently for naproxen, using molecularly imprinted polymer-coated multi-walled carbon nanotubes (MIP-MWCNTs) [37]. In this case, the MIP-MWCNTs were suspended in octanol, and this suspension was used as SLM. While the MIP-MWCNTs enhanced the mass transfer of naproxen across the SLM based on molecular recognition, sodium diclofenac which represented a similar chemical structure was extracted less. Thus, the MIP-MWCNTs enhanced the selectivity of the extraction system for the target analyte. Implementation of MIP-based selectivity represents another approach to specificity tuning in EME, and should be investigated further.
In several reports published in 2016, graphene based nano-sorbents were added to the SLM [36], [41], [42]. In one paper, extraction of pramipexole from urine samples was improved with graphene oxide dispersed in the SLM solvent. This was explained by the strong sorptive characteristics of the graphene material, and served as an additional pathway for analyte mass transfer. In another paper, graphene oxide was used in two-phase EME for extraction of methamphetamine from biological samples followed by gas chromatography [41]. Also in this case, graphene oxide was found to enhance the analyte mass transfer. Such positive effect of graphene oxide nano-sorbents was further supported by EME of chloramphenicol from dairy products [42]. In a fourth paper, nitrogen doped graphene was tested as SLM additive [40]. This material was dispersed into octanol, and served as cationic carrier for enhanced extraction of negatively charged naproxen and diclofenac from urine. However, addition of such nano-sorbents may also have a negative impact on the extraction performance. This was reported for EME of tartrazine from food samples using different carbon nanotubes and molecularly imprinted polymers dispersed in the SLM [38]. In this report, mass transfer was reduced by the addition of nano-sorbents to the SLM, due to increased trapping of target analyte in the SLM. Thus, although addition of nano-sorbents is highly interesting, it appears to be a challenging approach and should be investigated in more detail.
2.2. New supporting materials for the SLM
Up to date, most EME experiments have been acquired with the SLM immobilized in porous polypropylene polymers. The polypropylene polymers have been in the format of hollow fibers or as flat sheet membranes, and mainly commercially available materials have been used. Thus, there have been only a few investigations into alternative materials. Recently, however, a new support was proposed based on 100 µm thickness sheets of acrylic nanofiber membrane manufactured by electrospinning [43]. An SLM comprising Aliquat 336 dissolved in octanol was immobilized in this support, and used to extract the highly polar acidic drugs such as nicotinic acid, amoxicillin, hippuric acid, and salicylic acid. Recoveries were in the range 60%–85% after 10 min of EME. This confirmed that other supporting materials than polypropylene may show a great potential in EME. New supporting materials for the SLM represent another important area for research in the future.
2.3. New sample additives improving mass transfer
While substantial efforts have been reported on optimizing the chemical conditions in the SLM, much less research has been reported on dissolution of additives in the sample, with the purpose of improving mass transfer. However, in several recent papers, surfactants have been added to the sample to improve extraction recoveries [36], [42], [44], [45]. In one paper, Triton X-114 was added to the sample for extraction of pramipexole from urine samples [36]. Triton X-114 is a non-ionic surfactant, and extraction of pramipexole increased with increased concentration of surfactant in the sample up to about 0.2 mM. The latter corresponds to the critical micelle concentration (CMC) value for Triton X-114, where micelles start to be formed. The addition of surfactant to the sample almost doubled the extraction recovery. Similar data were obtained for extraction of dicamba and 2,4-DB (anionic analytes) from water samples containing the non-ionic carrier Triton X-100 at concentration slightly below CMC [45]. The positive effect of adding surfactant to the sample was confirmed in the third paper, where alfentanil, sufentanil, and methadone were extracted by two-phase EME system followed by gas chromatography [44]. In this case, sodium dodecyl sulphate (SDS) was used as surfactant, and the optimal level in the sample was 0.02% (m/v). This is slightly below CMC, and SDS was hypothesized to accumulate in the SLM/sample interface, and enhanced the mass transfer of cationic analytes through ionic interactions. Also the use of cationic surfactants has been reported. Thus, cetyltrimethylammonium bromide (CTAB) was added to the sample for extraction of chloramphenicol, and mass transfer of negatively charged analyte molecules was improved through ionic interactions with the cationic surfactant which was located in the SLM/sample interface [42].
2.4. New technical configurations
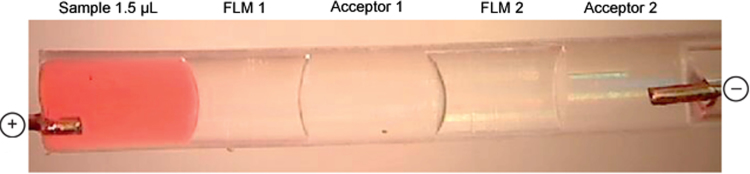
The majority of EME papers published up to date have utilized the hollow fibre configuration as illustrated in Fig. 1B. Recently, however, several research papers have discussed and introduced different technical variants of EME. In two papers, µ-EME has been developed with two individual free liquid membranes (FLMs) as illustrated in Fig. 3 [46], [47]. In this concept, EME was performed inside a small and transparent polymeric tubing. The different phases were contacting liquid plugs (1–5 μL), including the liquid membranes which were not immobilized in a porous support. In one paper, such five-phase μ-EME was developed for extraction and separation of cationic and anionic analytes into two separate acceptor solutions [47]. In this study, both drug substances and inorganic ions were tested as model analytes. In another paper, the five-phase μ-EME system was used for extraction and group separation of different basic drugs based on their pKa-values [46]. Although μ-EME was performed manually and in very simple and experimental set-up, the free liquid membrane (FLM) concept is highly interesting because it may be transferred to μ-chip systems in the future.
Fig. 3.
Photo of five-phase μ-EME system [46].
EME in μ-chip systems was known from earlier time, using SLMs immobilized in a porous polymeric support [48]. Such systems have also been reported in 2016 [49], [50]. In one paper, the sample was pumped through two individual EME chambers located in the same μ-chip [49]. In the first EME chamber, octanol was used as SLM, and acidic model analytes (mefenamic acid and diclofenac) were extracted into alkaline acceptor solution. The sample continued into the second EME chamber, where a basic model analyte (betaxolol) was extracted across NPOE as SLM and into acidic acceptor solution. Each EME chamber was controlled by a separate power supply, and the two extractions were optimized independently. A similar μ-chip was described for extraction of betaxolol, naltrexone, and nalmefene as model analytes [50]. The μ-chip was constructed from polymethyl methacrylate (PMMA), and the cross-sectional area of the flow channels was 200 µm×1000 µm. As mentioned above, there has been considerable interest in EME in μ-chip systems even before 2016 [48], and EME may develop to a very attractive sample preparation concept in this area. Thus, with implementation of EME, μ-chip systems may directly handle raw biological fluids such as whole blood in the future. We expect increased scientific activity in this area.
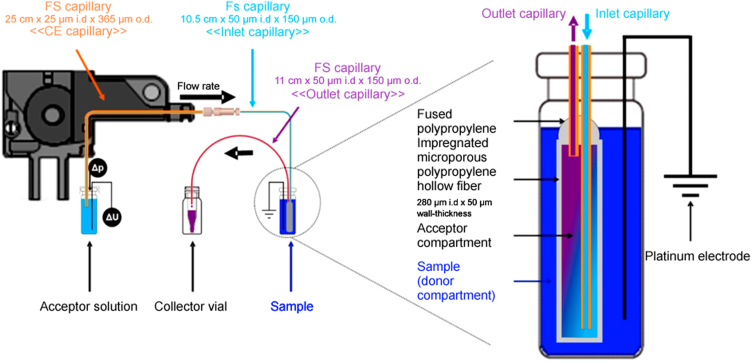
The μ-chip systems discussed above are dynamic systems, where the sample is pumped into the EME chamber. Another interesting approach to dynamic EME is illustrated in Fig. 4, where neuropeptides were used as model analytes [51]. This setup enabled continuous renewal of the acceptor solution, and it used a 50 µm thick porous support for the SLM. Renewal of the acceptor solution effectively eliminated any issues with drifting pH during extraction, which is especially critical in the acceptor solution. Because of the stable pH, partial back-extraction into the SLM was prevented. Consequently high enrichment and enhanced extraction recovery was obtained. The use of a very thin SLM also contributed to the high efficiency of the system.
Fig. 4.
Schematic illustration of dynamic EME setup [51].
In addition to the EME systems discussed above, several other technical variants of EME have been proposed in 2016. In one paper, extraction and group separation of basic and acidic analytes was performed [52]. The sample was split into two separate vials, one for extraction of basic analytes and the second for extraction of acidic analytes. The samples were stagnant, and naproxen, ibuprofen, and ketamine were selected as model analytes. In another paper, an EME-probe was developed for continuous extraction, and this was coupled directly to electrospray ionization mass spectrometry (ESI-MS) [53]. With this system, in-vitro drug metabolism was studied in real time; parent drug and metabolites were continuously extracted from the metabolic reaction mixture, and pumped into the MS-system for continuous measurement. The latter concept illustrates a type of application which is challenging to perform with traditional extraction methods.
While most EME has been conducted with a simple two-electrode system using platinum wires, a recent paper investigated a more sophisticated approach based on a rotating electrical field [54]. A central electrode was inserted in the acceptor solution inside the lumen of a hollow fibre, while five counter electrodes were located in the sample surrounding the SLM. An electrical circuit was designed to distribute the potential among the five counter electrodes in a rotating pattern. With this arrangement, recoveries increased by 50% as compared to traditional EME. Although the electrode system was more complicated, this paper illustrates that coupling of the electrical field is an important issue in EME, and probably the traditional use of two simple platinum wires is not always optimal.
The majority of EME papers published up to date have utilized static EME. For such systems, agitation is mandatory in order to maintain high extraction efficiency. Thus, static EME systems are either agitated or stirred. However, with the acceptor solution located inside the lumen of a hollow fibre of capillary dimensions, there is no or very little convection. Recently, this was improved using a rotating electrode in the acceptor solution [55]. This caused convection in the acceptor solution, and extraction efficiency increased as compared to traditional EME. This paper clearly demonstrated that traditional EME using hollow fibers may be limited in efficiency due to stagnant conditions in the acceptor solution. In another recent study, shaking and magnetic stirring were compared for EME operated in the hollow fibre configuration [56]. This study confirmed the importance of agitation in EME, and the results showed that shaking was more efficient than magnetic stirring. Also, the importance of stirring was found to increase with increased sample volume.
Automation of sample preparation is important for routine laboratories. Very recently, an automated EME system was developed in an HTC PAL autosampler and tuned for soft extraction of drug substances from in-vitro metabolic reaction mixtures [57]. The extraction probe was built into a Luer Lock adapter which was connected to an HTC PAL autosampler syringe. As the autosampler sucked sample, analytes were extracted into the lumen of the extraction probe and automatically transferred on-line to LC–MS analysis.
2.5. Theoretical understanding
Theoretical understanding is important in order to give EME a scientific anchor. Several papers on fundamental issues have been published before 2016, but additional papers have added important knowledge to this during 2016. In one paper, the fundamentals of EME were investigated using impedance measurements [58]. The results showed that the extraction of charged species can be impedometrically monitored, based on increase of the solution resistance and simultaneous decrease of the membrane resistance. The results also showed that electrical double layers were established at the SLM interfaces, and the double layer capacitance varied as function of extraction time.
In another study, pH effects in EME were investigated [59]. While basic drugs typically have been extracted with 10 mM hydrochloric acid as acceptor solution, improved EME performance was obtained in this work using 500 mM formic acid as alternative. With strong solution of formic acid, electrolytically induced variations in pH were suppressed, and therefore, back-extraction of analytes into the donor solution was avoided. Electrolytically produced H3O+ and OH− ions mostly remained in their corresponding solutions, and were not subject to transfer across the SLM. Therefore, high buffer capacity in the acceptor solution was mandatory to keep pH conditions stable. This is important knowledge, and preferable buffers should be used as acceptor solutions from this point forward.
As discussed above, the extraction current is important in EME, and should be kept as low as possible in order to avoid excessive electrolysis. A recent paper investigated extraction current in details [60]. In this paper, minimum recovery and repeatability occurred at the highest investigated magnitude of electrical current. Plots of extraction current versus extraction time showed completely different patterns under different extraction conditions, and several major operational parameters were found to influence the extraction current. Also, extraction current increased when extracting from complex matrices such as plasma and urine. Therefore, future optimization of operational parameters in EME should include work with real samples and measurements of extraction current.
2.6. New pharmaceutical applications
Up to date, the majority of EME applications have been pharmaceutical applications where different drug substances have been extracted from plasma, serum, whole blood, urine, and saliva. Also in 2016, new pharmaceutical EME applications were reported. In one very interesting paper, EME was combined with microcell UV–Vis spectrophotometeric detection for fast and sensitive determination of bismuth in bismuth subcitrate tablets and human plasma samples [61]. The working range of the assay was 2.1–800 ng/mL and detection limit for bismuth was approximately 1 ng/mL. Enrichment factors in the range 151–187 were obtained, and intra- and inter-day relative standard deviations were below 6.0%. The method was successfully applied for analysis of real samples.
Another forefront application was published on EME coupled with corona discharge ion mobility spectrometry (CD-IMS) for determination of antidepressant drugs (desipramine, sertraline, clomipramine, and citalopram) in urine samples [62]. EME was accomplished with NPOE as the SLM, with 190 V extraction potential, and with pH 4 in donor and pH 1 in acceptor solutions. Extractions were performed for 30 min with the whole assembly agitated at 1000 rpm. Under the optimized conditions, the proposed method provided linearity and repeatability data in compliance with FDA guidelines [63], detection limits in the range 0.9–1.5 ng/mL, pre-concentration factors in the range 158–190 times, and recoveries in the range 79%–95%.
In another study, EME was combined with HPLC–UV for quantification of four gonadotropin-releasing hormone agonist anticancer peptides (alarelin, leuprolide, buserelin and triptorelin) in biological samples [8]. The SLM comprised 95% of 1-octanol and 5% di-(2-ethylhexyl)-phosphate, the potential was 20 V, sample pH was 7.0, and acceptor solution was pH 1.0. After 15 min of extraction, extraction recoveries were 49%–71%, and the target analytes were pre-concentrated 82–118 times. Validation in the concentration range 2.0–1000 ng/mL was in accordance with FDA guidelines [63], and the method was successfully applied to human plasma samples.
In most publications up to date, EME has been combined with HPLC or LC-MS detection. However, EME is not limited to chromatography, and this was illustrated in a recent paper where EME was combined with differential pulse voltammetry (DPV) for determination of clozapine in human plasma samples [64]. Clozapine was extracted at 200 V from acidified sample and into a acidified acceptor solution. The latter was located in the lumen of a porous hollow fibre, and NPOE was used as the SLM. Three microelectrodes, an Ag/AgCl, a platinum wire, and a graphite pencil lead as the reference, counter, and working electrodes, respectively, were connected to the upper end of the hollow fibre. Under optimized conditions, the proposed method was linear in the range 3–1500 ng/mL (r2 =0.993). Extraction recovery was 42% and the pre-concentration factor was 114. The intra- and inter-day precisions were less than 3.5% RSD and 6.7% RSD, respectively. The proposed method was successfully applied for determination of clozapine in human plasma samples.
3. Where to go
As outlined in this paper, most EME research is currently focused on investigations into (a) new SLMs, (b) new technical configurations, (c) new applications, and (d) to some extent also fundamental aspects and understanding. This trend is expected to continue in the near future, and all new data are highly valuable for the progress of EME. However, EME will hopefully move gradually from being a pure research discipline and into a concept which will be used also for routine and commercial applications. For this to come, it is our view that the following issues have to be addressed:
-
•
More research into SLMs
-
•
More research into EME of larger biomolecules
-
•
More research into other challenging applications, where existing approaches are inappropriate
-
•
Development of commercially available and industrial standard EME equipment
More research into SLMs is required. As discussed above, general and stable SLMs are available for non-polar basic substances. A few new SLM candidates show potential for polar basic substances, but still SLMs for highly polar basic substances are missing. In addition, there are stability issues with the SLMs typically used for acidic substances, especially in contact with complex biological fluids. In order to combine EME with very simple detection systems, such as smartphones [65], there is also a need to develop SLMs with high specificity for priority substances. Such SLMs may incorporate elements of molecular recognition as discussed in Section 2.1.
Although several EME applications have been developed for peptides, more research into larger biomolecules is required. Analysis of large biomolecules is less mature as scientific discipline than analysis of small-molecule substances, and therefore, the former application area is more open to new technologies. Because most large biomolecules are charged, they are prone to electrokinetic migration and should be ideal candidates for EME. On the other hand, they are discriminated by the SLMs currently in use, and specialized SLMs for large biomolecules have to be developed. Other applications involving charged substances, where existing technologies are inappropriate, should also be targeted by EME. This may include continuous extractions and measurements in systems with rapid concentration fluctuations, or extractions from very small sample volumes/compartments.
Finally, commercially available equipment of industrial standard should be developed. Future EME conducted with commercially available equipment will definitely improve the quality, relevance, and impact of the research, and also open the door to routine and commercial laboratories. EME in 96-well system and in μ-chips may play an important role here.
4. Conclusions
Electromembrane extraction (EME) has been an active field of research for more than ten years, and the activity is increasing. The reason for this is linked to several unique conceptual properties. Most importantly, the selectivity can be controlled by an external power supply in combination with the chemical properties of the supported liquid membrane (SLM). There are no other extraction principles offering this type of flexibility. In addition, in EME the target analytes are finally collected in an aqueous solution, and EME represents a green chemistry approach to analytical extractions. Many EME papers up to date are very conceptual with preliminary data, but part of the research is now moving in direction of applications with performance, quality, and work-flow as required by routine and commercial laboratories. For EME to be established in the toolbox of these laboratories, development of commercial equipment is mandatory. This is currently in progress, and hopefully a product will be released in short time. With this equipment, the portfolio of SLMs should be extended, and applications should be developed where existing methods are inappropriate. In the current literature, there are several such examples, but this application platform has to be extended. Due to the unique selectivity of EME, the authors of this review are optimistic about the future for EME. Hopefully, more scientists will be inspired and contribute to the establishment of EME.
Acknowledgments
The Research Council of Norway is acknowledged for financial support through Grant 231917.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Pedersen-Bjergaard S., Rasmussen K.E. Electrokinetic migration across artificial liquid membranes - new concept for rapid sample preparation of biological fluids. J. Chromatogr. A. 2006;1109:183–190. doi: 10.1016/j.chroma.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen-Bjergaard S., Rasmussen K.E. Liquid–liquid–liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal. Chem. 1999;71:2650–2656. doi: 10.1021/ac990055n. [DOI] [PubMed] [Google Scholar]
- 3.Arthur C.L., Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990;62:2145–2148. [Google Scholar]
- 4.Jeannot M.A., Cantwell F.F. Solvent microextraction into a single drop. Anal. Chem. 1996;68:2236–2240. doi: 10.1021/ac960042z. [DOI] [PubMed] [Google Scholar]
- 5.Nilvé G., Audunsson G., Jönsson J.Å. Sample preparation by means of a supported liquid membrane for the determination of chlorophenoxyalkanoic acids. J. Chromatogr. A. 1989;471:151–160. [Google Scholar]
- 6.Huang C., Eibak L.E.E., Gjelstad A. Development of a flat membrane based device for electromembrane extraction: a new approach for exhaustive extraction of basic drugs from human plasma. J. Chromatogr. A. 2014;1326:7–12. doi: 10.1016/j.chroma.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Fotouhi L., Seidi S., Yamini Y. Evaluation of pulsed electromembrane extraction for the analysis of diclofenac and mefenamic acid in biological fluids. Anal. Method. 2015;7:2848–2854. [Google Scholar]
- 8.Nojavan S., Bidarmanesh T., Mohammadi A. Electromembrane extraction of gonadotropin-releasing hormone agonists from plasma and wastewater samples. Electrophoresis. 2016;37:826–833. doi: 10.1002/elps.201500555. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen-Bjergaard S., Rasmussen K.E. Electrical potential can drive liquid–liquid extraction for sample preparation in chromatography. Trends Anal. Chem. 2008;27:934–941. [Google Scholar]
- 10.Cabaleiro Domingueza N., Gjelstad A., Nadalc A.M. Selective electromembrane extraction at low voltages based on analyte polarity and charge. J. Chromatogr. A. 2012;1248:48–54. doi: 10.1016/j.chroma.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 11.Gjelstad A., Rasmussen K.E., Pedersen-Bjergaard S. Electrokinetic migration across artificial liquid membranes Tuning the membrane chemistry to different types of drug substances. J. Chromatogr. A. 2006;1124:29–34. doi: 10.1016/j.chroma.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Slampova A., Sindelar V., Kuban P. Application of a macrocyclic compound, bambus[6]uril, in tailor-made liquid membranes for highly selective electromembrane extractions of inorganic anions. Anal. Chim. Acta. 2017;950:49–56. doi: 10.1016/j.aca.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Middelthon-Bruer T.M., Gjelstad A., Rasmussen K.E. Parameters affecting electro membrane extraction of basic drugs. J. Sep. Sci. 2008;31:753–759. doi: 10.1002/jssc.200700502. [DOI] [PubMed] [Google Scholar]
- 14.Lee J., Khalilian F., Bagheri H. Optimization of some experimental parameters in the electro membrane extraction of chlorophenols from seawater. J. Chromatogr. A. 2009;1216:7687–7693. doi: 10.1016/j.chroma.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 15.Seidi S., Yamini Y., Saleh A. Electromembrane extraction of levamisole from human biological fluids. J. Sep. Sci. 2011;34:585–593. doi: 10.1002/jssc.201000735. [DOI] [PubMed] [Google Scholar]
- 16.Seip K.F., Gjelstad A., Pedersen-Bjergaard S. The potential of electromembrane extraction for bioanalytical applications. Bioanalysis. 2015;7:463–480. doi: 10.4155/bio.14.303. [DOI] [PubMed] [Google Scholar]
- 17.Kuban P., Strieglerova L., Gebauer P. Electromembrane extraction of heavy metal cations followed by capillary electrophoresis with capacitively coupled contactless conductivity detection. Electrophoresis. 2011;32:1025–1032. doi: 10.1002/elps.201000462. [DOI] [PubMed] [Google Scholar]
- 18.Kuban P., Slampova A., Bocek P. Electric field-enhanced transport across phase boundaries and membranes and its potential use in sample pretreatment for bioanalysis. Electrophoresis. 2010;31:768–785. doi: 10.1002/elps.200900561. [DOI] [PubMed] [Google Scholar]
- 19.Petersen N.J., Rasmussen K.E., Pedersen-Bjergaard S. Electromembrane extraction from biological fluids. Anal. Sci. 2011;27:965–972. doi: 10.2116/analsci.27.965. [DOI] [PubMed] [Google Scholar]
- 20.Gjelstad A., Pedersen-Bjergaard S. Electromembrane extraction: a new technique for accelerating bioanalytical sample preparation. Bioanalysis. 2011;3:787–797. doi: 10.4155/bio.11.13. [DOI] [PubMed] [Google Scholar]
- 21.Marothu V.K., Gorrepati M., Vusa R. Electromembrane extraction—a novel extraction technique for pharmaceutical, chemical, clinical and environmental analysis. J. Chromatogr. Sci. 2013;51:619–631. doi: 10.1093/chromsci/bmt041. [DOI] [PubMed] [Google Scholar]
- 22.Gjelstad A., Pedersen-Bjergaard S. Recent developments in electromembrane extraction. Anal. Method. 2013;5:4549–4557. [Google Scholar]
- 23.Lindenburg P.W., Ramautar R., Hankemeier Thomas. The potential of electrophoretic sample pretreatment techniques and new instrumentation for bioanalysis, with a focus on peptidomics and metabolomics. Bioanalysis. 2013;5:2785–2801. doi: 10.4155/bio.13.254. [DOI] [PubMed] [Google Scholar]
- 24.Gjelstad A., Pedersen-Bjergaard S. Electromembrane extraction – three-phase electrophoresis for future preparative applications. Electrophoresis. 2014;35:2421–2428. doi: 10.1002/elps.201400127. [DOI] [PubMed] [Google Scholar]
- 25.Gjelstad A., Pedersen-Bjergaard S., Seip K.F. Electromembrane extraction as a rapid and selective miniaturized sample preparation technique for biological fluids. Bioanalysis. 2015;7:2203–2209. doi: 10.4155/bio.15.150. [DOI] [PubMed] [Google Scholar]
- 26.Seip K.F., Gjelstad A., Pedersen-Bjergaard S. The potential of electromembrane extraction for bioanalytical applications. Bioanalysis. 2015;7:463–480. doi: 10.4155/bio.14.303. [DOI] [PubMed] [Google Scholar]
- 27.Huang C., Seip K.F., Gjelstad A. Electromembrane extraction for pharmaceutical and biomedical analysis – Quo vadis. J. Pharm. Biomed. Anal. 2015;113:97–107. doi: 10.1016/j.jpba.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Huang C., Jensen H., Seip K.F. Mass transfer in electromembrane extraction – the link between theory and experiments. J. Sep. Sci. 2016;39:188–197. doi: 10.1002/jssc.201500905. [DOI] [PubMed] [Google Scholar]
- 29.Rezazadeh M., Yamini Y., Seidi S. Electrically stimulated liquid-based extraction techniques in Bioanalysis. Bioanalysis. 2016;8:815–828. doi: 10.4155/bio.16.23. [DOI] [PubMed] [Google Scholar]
- 30.Oedit A., Ramautar R., Hankemeier T. Electroextraction and electromembrane extraction: advances in hyphenation to analytical techniques. Electrophoresis. 2016;37:1170–1186. doi: 10.1002/elps.201500530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C., Gjelstad A., Pedersen-Bjergaard S. Organic solvents in electromembrane extraction: recent insights. Rev. Anal. Chem. 2016;35:169–183. [Google Scholar]
- 32.Strieblerova L., Kuban P., Bocek P. Electromembrane extraction of amino acids from body fluids followed by capillary electrophoresis with capacitively coupled contactless conductivity detection. J. Chromatogr. A. 2018;2011:6248–6255. doi: 10.1016/j.chroma.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Østegaard Kjelsen I.J., Gjelstad A., Rasmussen K.E. Low-voltage electromembrane extraction of basic drugs from biological samples. J. Chromatogr. A. 2008;1180:1–9. doi: 10.1016/j.chroma.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Seip K.F., Stigsson J., Gjelstad A. Electromembrane extraction of peptides – fundamental studies on the supported liquid membrane. J. Sep. Sci. 2011;34:3410–3417. doi: 10.1002/jssc.201100558. [DOI] [PubMed] [Google Scholar]
- 35.Huang C., Gjelstad A., Pedersen-Bjergaard S. Electromembrane extraction with alkylated phosphites and phosphates as supported liquid membranes. J. Membr. Sci. 2017;526:18–24. [Google Scholar]
- 36.Fashi A., Khanban F., Yaftian M.R. The cooperative effect of reduced graphene oxide and Triton X-114 on the electromembrane microextraction efficiency of Pramipexole as a model analyte in urine samples. Talanta. 2017;162:210–217. doi: 10.1016/j.talanta.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 37.Tahmasebi Z., Hosseiny Davarani S.S., Asgharinezhad A.A. An efficient approach to selective electromembrane extraction of naproxen by means of molecularly imprinted polymer-coated multi-walled carbon nanotubes-reinforced hollow fibers. J. Chromatogr. A. 2016;1470:19–26. doi: 10.1016/j.chroma.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 38.Yaripour S., Mohammadi A., Nojavan S. Electromembrane extraction of tartrazine from food samples: effects of nano-sorbents on membrane performance. J. Sep. Sci. 2016;39:2642–2651. doi: 10.1002/jssc.201600071. [DOI] [PubMed] [Google Scholar]
- 39.Huang C., Seip K.F., Gjelstad A. Electromembrane extraction of polar basic drugs from plasma with pure bis(2-ethylhexyl) phosphite as supported liquid membrane. Anal. Chim. Acta. 2016;934:80–87. doi: 10.1016/j.aca.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Atarodi A., Chamsaz M., Moghaddam A.Z. Introduction of high nitrogen doped graphene as a new cationic carrier in electromembrane extraction. Electrophoresis. 2016;37:1191–1200. doi: 10.1002/elps.201600001. [DOI] [PubMed] [Google Scholar]
- 41.Bagheri H., Zavareh A.F., Koruni M.H. Graphene oxide assisted electromembrane extraction with gas chromatography for the determination of methamphetamine as a model analyte in hair and urine samples. J. Sep. Sci. 2016;39:1182–1188. doi: 10.1002/jssc.201501209. [DOI] [PubMed] [Google Scholar]
- 42.Fashi A., Khanban F., Yaftian M.R. Improved electromembrane microextraction efficiency of chloramphenicol in dairy products: the cooperation of reduced graphene oxide and a cationic surfactant. RSC Adv. 2016;6:112748–112755. [Google Scholar]
- 43.Román-Hidalgo C., Martín-Valero M.J., Fernández-Torres R. New nanostructured support for carrier-mediated electromembrane extraction of high polar compounds. Talanta. 2016;162:32–37. doi: 10.1016/j.talanta.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Zahedi P., Hosseiny Davarani S.S., Moazamo H.R. Surfactant assisted pulsed two-phase electromembrane extraction followed by GC analysis for quantification of basic drugs in biological samples. J. Pharm. Biomed. Anal. 2016;117:485–491. doi: 10.1016/j.jpba.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Bagheri H., Fakhari A.R., Sahragard A. A novel strategy based on surfactant assisted electromembrane extraction for the determination of dicamba and 2,4-DB as model herbicides in real water samples. RSC Adv. 2016;6:4843–4849. [Google Scholar]
- 46.Kuban P., Seip K.F., Gjelstad A. Micro-electromembrane extraction using multiple free liquid membranes and acceptor solutions - Towards selective extractions of analytes based on their acid-base strength. Anal. Chim. Acta. 2016;943:64–73. doi: 10.1016/j.aca.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 47.Kuban P., Bocek P. Simultaneous micro-electromembrane extractions of anions and cations using multiple free liquid membranes and acceptor solutions. Anal. Chim. Acta. 2016;908:113–120. doi: 10.1016/j.aca.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Petersen N.J., Jensen H., Honore Hansen S. On-chip electromembrane extraction. Microfluid. Nanofluid. 2010;9:881–888. [Google Scholar]
- 49.Asl Y.A., Yamini Y., Seidi S. Simultaneous extraction of acidic and basic drugs via on-chip electromembrane extraction. Anal. Chim. Acta. 2016;937:61–68. doi: 10.1016/j.aca.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 50.Asl Y.A., Yamini Y., Seidi S. A novel approach to the consecutive extraction of drugs with different properties via on chip electromembrane extraction. Analyst. 2016;141:311–318. doi: 10.1039/c5an02019k. [DOI] [PubMed] [Google Scholar]
- 51.Drouin N., Rudaz S., Schappler J. Dynamic-electromembrane extraction: a technical development for the extraction of neuropeptides. Anal. Chem. 2016;88:5308–5315. doi: 10.1021/acs.analchem.6b00559. [DOI] [PubMed] [Google Scholar]
- 52.Nojavan S., Asadi S. Electromembrane extraction using two separate cells: a new design for simultaneous extraction of acidic and basic compounds. Electrophoresis. 2016;37:587–594. doi: 10.1002/elps.201500455. [DOI] [PubMed] [Google Scholar]
- 53.Fuchs D., Gabel-Jensen C., Jensen H. Direct coupling of a flow-flow electromembrane extraction probe to LC-MS. Anal. Chim. Acta. 2016;905:93–99. doi: 10.1016/j.aca.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Hosseiny Davarani S.S., Moazami H.R., Memarian E. Electromembrane extraction through a virtually rotating supported liquid membrane. Electrophoresis. 2016;37:339–346. doi: 10.1002/elps.201500296. [DOI] [PubMed] [Google Scholar]
- 55.Asadi S., Tabani H., Khodaei K. Rotating electrode in electro membrane extraction: a new and efficient methodology to increase analyte mass transfer. RSC Adv. 2016;6:101869–101879. [Google Scholar]
- 56.Nojavan S., Tahmasebi Z., Hosseiny Davarani S.S. Effect of type of stirring on hollow fiber liquid phase microextraction and electromembrane extraction of basic drugs: speed up extraction time and enhancement of extraction efficiency. RSC Adv. 2016;6:110221–110228. [Google Scholar]
- 57.Fuchs D., Pedersen-Bjergaard S., Jensen H. Fully automated electro membrane extraction autosampler for LC–MS systems allowing soft extractions for high-throughput applications. Anal. Chem. 2016;88:6797–6804. doi: 10.1021/acs.analchem.6b01243. [DOI] [PubMed] [Google Scholar]
- 58.Mohammadi J., Hosseiny Davarani S.S., Moazami H.R. Impedometric monitoring of the behavior of the supported liquid membrane in electromembrane extraction systems: an insight into the origin of optimized experimental parameters. Anal. Chim. Acta. 2016;934:98–105. doi: 10.1016/j.aca.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Slampova A., Kuban P., Bocek P. Additional considerations on electrolysis in electromembrane extraction. J. Chromatogr. A. 2016;1429:364–368. doi: 10.1016/j.chroma.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 60.Rahmani T., Rahimi A., Nojavan S. Study on electrical current variations in electromembrane extraction process: relation between extraction recovery and magnitude of electrical current. Anal. Chim. Acta. 2016;903:81–90. doi: 10.1016/j.aca.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 61.Fashi A., Reza Yaftian M., Zamani A. Electromembrane-microextraction of bismuth in pharmaceutical and human plasma samples: optimization using response surface methodology. Microchem. Journ. 2017;130:71–78. [Google Scholar]
- 62.Aladaghlo Z., Fakhari A.R., Hasheminasab K.S. Application of electromembrane extraction followed by corona discharge ion mobility spectrometry analysis as a fast and sensitive technique for determination of tricyclic antidepressants in urine samples. Microchem. Journ. 2016;129:41–48. [Google Scholar]
- 63.〈https://www.fda.gov/downloads/Drugs/Guidances/ucm368107.pdf〉.
- 64.Rouhollahi A., Kouchaki M., Seidi S. Electrically stimulated liquid phase microextraction combined with differential pulse voltammetry: a new and efficient design for in situ determination of clozapine from complicated matrices. RSC Adv. 2016;6:12943–12952. [Google Scholar]
- 65.Seidi S., Rezazadeh M., Yamini Y. Low voltage electrically stimulated lab-on-a-chip device followed by red-green-blue analysis: a simple and efficient design for complicated matrices. Analyst. 2014;139:5531–5537. doi: 10.1039/c4an01124d. [DOI] [PubMed] [Google Scholar]