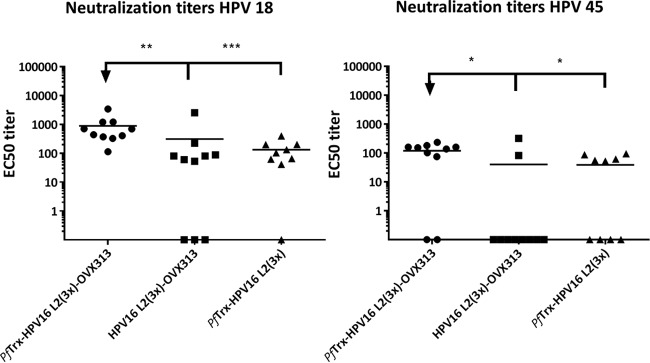
FIG 2.
Inclusion of the OVX313 heptamerization domain in the vaccine formulation increases immunogenicity of Trx-L2 antigens. HPV18 and HPV45 L1-PBNA with sera collected 1 month after the last immunization of mice with PfTrx HPV16 L2(20–38)3 (-OVX313) and HPV16 L2(20–38)3 (-OVX313) antigens formulated with alum-MPLA. PfTrx HPV16 L2(20–38)3 was used as a control. Symbols represent individual neutralization titers of immune sera; geometric means of the titers for each group are indicated by horizontal lines. A P value of ≤0.05 was considered significant. *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001.