Abstract
Metabolic acidosis is not uncommon in CKD and is linked with bone demineralization, muscle catabolism, and higher risks of CKD progression and mortality. Clinical practice guidelines recommend maintaining serum total CO2 at ≥22 mEq/L to help prevent these complications. Although a definitive trial testing whether correcting metabolic acidosis improves clinical outcomes has not been conducted, results from small, single-center studies support this notion. Furthermore, biologic plausibility supports the notion that a subset of patients with CKD have acid-mediated organ injury despite having a normal serum total CO2 and might benefit from oral alkali before overt acidosis develops. Identifying these individuals with subclinical metabolic acidosis is challenging, but recent results suggest that urinary acid excretion measurements may be helpful. The dose of alkali to provide in this setting is unknown as well. The review discusses these topics and the prevalence and risk factors of metabolic acidosis, mechanisms of acid-mediated organ injury, results from interventional studies, and potential harms of alkali therapy in CKD.
Keywords: chronic metabolic acidosis, chronic kidney disease, ESRD, acidosis, mortality
Metabolic acidosis was one of the first recognized complications of kidney failure. Landmark studies identified the importance of reduced renal ammonia production in the pathogenesis of acidosis and effects of acidosis on bone demineralization and protein catabolism.1–6 These consequences informed the clinical practice guideline recommendation to treat metabolic acidosis with alkali in CKD.7,8 Results from single-center studies suggest that correcting acidosis may also preserve kidney function in CKD.9–11 Although these potential benefits require confirmation in a definitive clinical trial, there is accumulating evidence that alkali might preserve kidney function in patients with CKD with normal serum total CO2 (tCO2) as well.12–14 If true, this would lead to a significant paradigm shift in how alkali is utilized in CKD. However, it is unclear which patients with normal tCO2 are most likely to benefit from alkali or the dose to prescribe in this setting. The prevalence and risk factors of metabolic acidosis, mechanisms of acid-mediated organ injury, results from interventional studies, and potential harms of alkali therapy are reviewed.
Prevalence and Risk Factors of Overt Metabolic Acidosis in CKD
Most nondialysis-requiring patients with CKD do not have metabolic acidosis, largely because of compensatory renal ammonia production and bone buffering.4,6 When defined as a serum tCO2 <22 mEq/L, the prevalence of metabolic acidosis is 15%.15,16 However, its prevalence increases with worsening kidney function. For example, the prevalence of acidosis was 7% in stage 2, 13% in stage 3, and 37% in stage 4 CKD Chronic Renal Insufficiency Cohort Study (CRIC) participants,16 and similar trends were observed in the NephroTest Cohort.15
Reduced GFR is the most important risk factor for acidosis (Table 1).16 Related to GFR is the capacity to excrete acid. For example, African American Study of Kidney Disease and Hypertension (AASK) participants had 2.5-fold higher risk of incident metabolic acidosis if ammonium excretion was <15 mEq/d.17 The quantity of acid-producing (protein, grains, cheese) and alkali-producing (fruits and vegetables) foods consumed contributes as well.10,18 Renin-angiotensin-aldosterone system (RAAS) inhibitors lower tCO2 by attenuating aldosterone-mediated acid secretion,19 and hyperkalemia, irrespective of RAAS inhibition, lowers tCO2 by reducing kidney ammoniagenesis.20 On the other hand, diuretics increase serum tCO2. Hence, a number of factors affect tCO2; however, kidney function and acid excretion capacity are key determinants of metabolic acidosis in CKD.
Table 1.
Risk factors for overt metabolic acidosis in CKD after multivariate adjustment
| Risk Factor | Comments |
|---|---|
| Lower GFR | 2-fold higher risk for stage 3 and 7-fold higher risk for stage 4 (compared with stage 2 CKD) |
| Lower ammonium excretion | 2.5-fold higher risk of incident acidosis if <15 mEq/d (compared with >25 mEq/d) |
| Hyperkalemia | 2.4-fold higher risk if ≥5.0 mEq/L |
| Albuminuria | 2-fold higher risk if ≥30 mg/gm |
| Smoking | 43% higher risk |
| Anemia | 40% higher risk |
| Higher serum albumin | 35% higher risk for each 1 gm/dl increase |
| Diuretic use | 30% lower risk |
| Use of ACE-i/ARB | 24% higher risk |
Effects of Metabolic Acidosis on Bone and Muscle in CKD
The contribution of metabolic acidosis to bone demineralization and protein catabolism were cited by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines as reasons to maintain serum tCO2 ≥22 mEq/L using oral alkali as necessary in CKD (Figure 1A),7,8 a recommendation subsequently supported by Kidney Disease: Improving Global Outcomes guidelines.21 Landmark studies demonstrated that renal insufficiency stimulates skeletal muscle proteolysis through acidification-dependent ubiquitination and that bone releases calcium carbonate to buffer acid leading to hypercalciuria and loss of bone mineral.2–6 In vivo studies also showed that extracellular acidification increases activity of osteoclasts and inhibits activity of osteoblasts.22
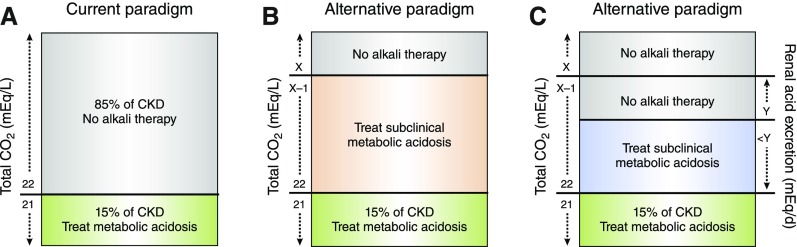
Figure 1.
Urinary acid excretion may identify patients with CKD and normal tCO2 who have subclinical metabolic acidosis. (A) The current metabolic acidosis treatment paradigm is to withhold alkali until serum tCO2 falls below 22 mEq/L, in which case only 15% of patients with CKD receive alkali therapy. (B) An alternative paradigm assumes that patients with CKD and tCO2 below a threshold value (represented as X), have either overt acidosis or subclinical metabolic acidosis and are potential candidates for alkali therapy. (C) Another possible approach would be to treat patients with CKD with tCO2<22 mEq/L and those with subclinical metabolic acidosis defined by a normal tCO2 and urinary acid excretion below a threshold value (represented as Y).
Although it is biologically plausible that treatment of metabolic acidosis improves musculoskeletal health in CKD, evidence from interventional studies is limited to a few studies with relatively few participants. With respect to muscle, sodium bicarbonate treatment improved sit-stand time in a single-arm study of 20 patients with CKD with serum tCO2 20–24 mEq/L; however, there was no effect on hand grip strength.23 Correction of acidosis with sodium bicarbonate (n=67) improved mid-arm muscle circumference, suggesting increased muscle mass, compared with usual care (n=67) over 2 years.9 In terms of bone health, treatment of metabolic acidosis (n=20) attenuated parathyroid hormone elevations over 3 months as compared with untreated patients with metabolic acidosis (n=20).24 Hence, evidence that treatment of metabolic acidosis improves musculoskeletal health in CKD is sparse. In kidney transplant recipients, treatment with potassium citrate (n=19) normalized low tCO2 and improved bone histology and turnover markers compared with potassium chloride over 1 year (n=11), and in ESRD, correction of acidosis ameliorated protein catabolism and improved bone histology.25–29 Nevertheless, results from these non-CKD populations should be cautiously applied to those with CKD, and better evidence that treatment of metabolic acidosis improves musculoskeletal health is needed.
Effects of Nonvolatile Acids on the Kidney
Maintenance of normal systemic tCO2 and pH is a principal kidney function. However, kidney compensatory mechanisms to maintain normal acid-base homeostasis in response to nonvolatile acids may cause further kidney injury in CKD. In the healthy state, kidneys respond to these acids by enhancing urinary acid excretion, mainly as ammonium.30 With reduced kidney function, total urinary ammonium excretion declines, but ammonia production per nephron markedly increases to accommodate nephron loss and ongoing acid exposure.1,17,31,32 This compensatory response to facilitate acid excretion leads to high local intrarenal concentrations of ammonia. This detrimentally activates the alternative pathway of complement through nucleophilic disruption of the internal thioester bond of the complement protein C3 by lone electron pairs on NH3 leading to a cascade of events that ultimately promotes tubulointerstitial fibrosis.33 This mechanism of kidney injury was observed in animals with subtotal nephrectomy and animals with normal renal mass and hypokalemia, and alkali treatment attenuated kidney injury in both models by suppressing kidney ammoniagenesis.34,35 In humans, treatment of metabolic acidosis reduced urine, but not serum, levels of complement activation proteins, suggesting that intrarenal complement activation was ameliorated by lowering kidney ammoniagenesis.36 However, a direct link between intrarenal ammonia and kidney fibrosis in humans has yet to be established.
Upregulation of systemic and kidney endothelin-1 (ET-1) levels is another compensatory response to nonvolatile acids that helps maintain normal tCO2 and pH but promotes kidney injury. ET-1 facilitates acid excretion by stimulating proximal and distal Na+/H+ exchange, reducing distal bicarbonate secretion through nitric oxide, and stimulating adrenal aldosterone release to facilitate H+-ATPase activity.37,38 However, ET-1 promotes kidney injury, proteinuria, inflammation, and kidney fibrosis,39 and endothelin A receptor antagonism prevented GFR decline in subtotal nephrectomy animals on an acid-producing diet.40
In addition to ET-1–mediated aldosterone upregulation, systemic and kidney angiotensin II levels are increased in subtotal nephrectomy animals with normal tCO2 but interstitial acid accumulation, determined by microdialysis of the kidney cortex. Eliminating kidney interstitial acid accumulation with sodium bicarbonate reduced angiotensin II levels and preserved GFR better than angiotensin II receptor blockade, suggesting that amelioration of acid accumulation exerts renoprotective effects above and beyond angiotensin II inhibition.41,42 Thus, compensatory upregulation of kidney ammonia production and ET-1 and RAAS activity to preserve systemic tCO2 and pH are hypothesized to be detrimental in CKD. Interstitial acid accumulation may also directly contribute to kidney injury, along with intrarenal inflammation, insulin resistance, and oxidative stress.43–47
Subclinical Metabolic Acidosis in CKD
These compensatory responses to nonvolatile acids suggest that acid-mediated kidney injury might be occurring even in the setting of normal tCO2 in CKD. This state of nonvolatile acid–mediated organ injury despite having a normal tCO2 has been referred to as eubicarbonatemic metabolic acidosis,48 preclinical metabolic acidosis,49 or subclinical metabolic acidosis.50,51 In persons with preserved kidney function, subclinical metabolic acidosis might be observed in conditions such as chronic diarrhea or high dietary protein intake.48 In these cases, enhanced urinary ammonium excretion might identify individuals with preserved kidney function who have subclinical metabolic acidosis despite having normal serum tCO2.48 A low-normal tCO2 might be a sign of subclinical metabolic acidosis as well (Figure 1B). In AASK, United States veterans with CKD, and patients with CKD at Cleveland Clinic Foundation, those with low-normal tCO2 had higher risk of mortality or GFR decline than those with high-normal tCO2.52–54 Conceivably, those with low-normal tCO2 have higher risk of these outcomes because they require a greater compensatory response to maintain normal tCO2 than those with high-normal tCO2 and therefore have more acid-mediated organ injury.
Alternatively, urinary acid excretion measurements might aid in the diagnosis of subclinical metabolic acidosis in CKD. Higher urinary acid excretion in CKD might signal a high-acid diet. On the other hand, lower urinary acid excretion in CKD might indicate impaired tubular capacity to produce and excrete acid with tissue acid accumulation. Hence, a u-shaped relationship between urinary acid excretion and adverse outcomes might be expected. However, in the CRIC study, the NephroTest Cohort, and AASK, lower, but not higher, urinary acid excretion was associated with higher risk of ESRD or death.17,32,55 In AASK, those with tCO2≥22 mEq/L and lower ammonium excretion had (1) higher risk of ESRD or death than those with tCO2≥22 mEq/L and higher ammonium excretion and (2) 2.5-fold higher risk of incident acidosis at 1 year, consistent with low urinary ammonium excretion signaling impaired acid excretion with acid retention rather than a low-acid or high-alkali diet or metabolic alkalosis.17 Thus, quantification of urinary acid excretion, particularly ammonium, may be a useful guide to identify those with subclinical metabolic acidosis but at high risk of overt metabolic acidosis and poor outcomes (Figure 1C).
Other urine measurements may identify patients with CKD with subclinical metabolic acidosis. Higher net endogenous acid production calculated from urine urea nitrogen and potassium measurements was associated with GFR decline in AASK.56 Urine sulfate and citrate levels have also been proposed.48 Urine sulfate is largely an indicator of animal protein intake48; hence, higher urine sulfate levels might be expected to associate with worse outcomes in CKD. However, higher urine sulfate was associated with lower risk of mortality in kidney transplant recipients and lower risk of renal events in diabetic nephropathy.57,58 Hence, its utility as an index of dietary acid–related subclinical metabolic acidosis in CKD is uncertain. Quantification of urinary citrate levels might also be informative, because citrate excretion decreases in response to metabolic acidosis59; however, a relationship between urinary citrate and clinical outcomes in CKD has not been reported. These are possible ways of defining subclinical metabolic acidosis in CKD; however, the best way to identify these individuals is not yet certain.
Alkali May Preserve GFR in Patients with CKD and Subclinical Metabolic Acidosis
Results from alkali interventional studies in hypertensive patients with CKD with normal tCO2 support the notion that subclinical metabolic acidosis causes kidney injury. In stage 2 hypertensive patients with CKD with mean tCO2 approximately 26 mEq/L, those treated with sodium bicarbonate (n=40) had higher GFR than those in sodium chloride (n=40) and placebo arms (n=40) after 5 years, despite having similar baseline GFR.14 Similarly, treatment with either sodium bicarbonate or fruits and vegetables better preserved GFR than usual care in stage 3 hypertensive patients with CKD with tCO2 22–24 mEq/L over 3 years.12 Finally, 30 days of fruits and vegetables or sodium bicarbonate lowered albuminuria and other kidney injury markers in hypertensive stage 2, but not stage 1, patients with CKD with tCO2>24.5 mEq/L.13 The effects of oral alkali on bone in patients with CKD with normal tCO2 have not been evaluated. However, in persons without CKD, oral alkali reduces urinary calcium excretion and plasma C-terminal crosslinking telopeptide of type 1 collagen (CTX) levels and increases bone mineral density.60–62
Despite the promising results and biologic plausibility, the use of pharmacologic alkali in patients with CKD with normal tCO2 is not recommended unless results from a well designed, multicenter trial are supportive. Results from several ongoing studies evaluating pleiotropic effects of sodium bicarbonate in patients with CKD with normal tCO2 will provide additional insight.63–65 One of the main challenges moving forward is that the dose of alkali to prescribe in patients with normal tCO2 is uncertain. In metabolic acidosis, alkali is typically titrated until the serum tCO2 is ≥22 mEq/L. In those with normal tCO2, weight-based sodium bicarbonate doses of 0.3–0.5 mEq/kg per day have been administered.12,14,63,64 Nevertheless, sodium bicarbonate seems to be safe in this dose range and raises the possibility that higher doses might be more efficacious and safe in CKD. In a 6-week dose-escalation study of 20 patients with stage 3b/4 CKD with a tCO2 of 20–24 mEq/L, sodium bicarbonate doses up to 1 mEq/kg per day were well tolerated and improved quadricep strength.23 Whether a dose in this range is safe and tolerable over a longer period is uncertain. This is the overarching goal of the CKD Pilot Studies Consortium Bicarbonate Administration to Stabilize eGFR Pilot Study. Briefly, 192 patients with CKD with a tCO2 of 20–28 mEq/L will receive one of two doses of sodium bicarbonate (0.5 or 0.8 mEq/kg daily) or placebo over 28 weeks. The main objectives are to determine the safety, tolerability, and compliance of these doses as well as the effect on urine ammonium excretion as a pharmacodynamics assessment.65 Another challenge moving forward is that it is not clear how best to identify patients with normal tCO2 who would be most likely to benefit during a clinical trial of reasonable duration. Urinary acid excretion measurements may be helpful as alluded to, but none of the published or ongoing studies have used these to select participants. This may be important to consider in future interventional studies.
Alkali May Preserve Kidney Function in Patients with CKD and Overt Metabolic Acidosis
In an open-label study of 134 participants with CKD stage 4/5 and metabolic acidosis, de Brito-Ashurst et al.9 found that treatment of acidosis by titrating sodium bicarbonate toward a goal tCO2 of ≥23 mEq/L slowed GFR decline and reduced the risk of ESRD. Phisitkul et al.11 reported results from a nonrandomized, open-label comparison of hypertensive patients with CKD with metabolic acidosis who tolerated sodium citrate (n=30) to similar individuals who were intolerant of sodium citrate and sodium bicarbonate (n=29), and those who tolerated sodium citrate had less GFR decline. Nutritional therapy with fruits and vegetables (n=36) to lower the potential renal acid load by 50% led to similar reductions in kidney injury markers at 1 year as 1.0 mEq/kg daily of sodium bicarbonate (n=35) in stage 4 hypertensive patients with CKD with metabolic acidosis and serum potassium <4.6 mEq/L.10 Although the lower alkali dose with fruits and vegetables raised tCO2 less than sodium bicarbonate, it lowered BP more.10 The accompanying dietary fiber with fruits and vegetables may also explain similar changes in these markers despite the lower alkali dose.66,67 Importantly, serum potassium did not increase substantially in the fruits and vegetables arm.10
The results from these single-center studies support the possibility that nutritional and pharmacologic treatment of overt metabolic acidosis preserves renal function in CKD. Although clinical practice guidelines have recommended treatment of metabolic acidosis in CKD for nearly two decades now, a large-scale, well designed clinical trial testing pleiotropic effects of correcting metabolic acidosis on muscle, bone, and kidney health in CKD remains necessary.
Is Sodium-Based Alkali Safe?
Pharmacologic management of metabolic acidosis using potassium-based alkali may cause hyperkalemia. Hence, sodium-based alkali is commonly prescribed in CKD. Given the potential of sodium-related fluid retention, fluid weight gain, worsening BP, peripheral edema, and pulmonary congestion, an important consideration is whether sodium-based alkali is safe in CKD. Several lines of evidence suggest that these fluid-related possibilities are more likely to occur when sodium is accompanied by the chloride anion rather than bicarbonate or citrate.68,69 Although the mechanisms for this are beyond the scope of this review, hyperchloremia induces tubuloglomerular feedback, renal vasoconstriction, and, consequently, reduced GFR.70,71
The apparent safety of sodium-based alkali is thus far supported by evidence from the previously mentioned clinical studies. In the study by de Brito-Ashurst et al.,9 systolic BP was similar between the sodium bicarbonate and the usual care groups at baseline and during follow-up, and although a higher percentage of sodium bicarbonate–treated participants required an increase in antihypertensive therapy (61% versus 48%; P=0.17) and loop diuretic therapy (39% versus 30%; P=0.50), the differences were not statistically significant. This suggests that BP was manageable with sodium bicarbonate. More importantly, none in the sodium bicarbonate arm discontinued treatment or were hospitalized for congestive heart failure (CHF).9 In the study by Phisitkul et al.,40 there was no suggestion that systolic BP was higher in the sodium citrate group than the control group. No information about hospitalizations was mentioned and no participants died during the 2-year follow-up. These results suggest that sodium-based alkali is relatively safe in terms of fluid-related adverse effects. Uncontrolled BP (>150/90 mm Hg), CHF, or morbid obesity were exclusion criteria in the study by de Brito-Ashurst et al.9 BP was controlled before exposure to sodium citrate and known cardiovascular disease, peripheral edema, liver failure, or nephrotic syndrome were exclusion criteria in the study by Phisitkul et al.40 The safety of sodium-based alkali in patients with these comorbidities needs further study.
Potential Cardiovascular Harms of Raising Serum tCO2
Although sodium-based alkali has not raised serious safety concerns in CKD so far, raising tCO2 may have adverse cardiovascular effects. First, tCO2>26 mEq/L was associated with higher risk of incident CHF in two CKD cohorts.72,73 Whether raising tCO2 toward these levels causes CHF is uncertain. Second, studies in animal models of CKD suggest that metabolic acidosis might prevent vascular calcification.74,75 Hence, correcting acidosis might promote or worsen vascular calcification in CKD. To date, these possibilities remain theoretic, but a well designed, long-term clinical trial is needed to evaluate the overall risk and benefits of sodium-based alkali in CKD.
Dietary Therapy of Metabolic Acidosis in CKD
Dietary strategies incorporating fruits and vegetables should be considered in the management of metabolic acidosis in CKD and are reasonable for those with normal tCO2. In addition to providing base, the accompanying potassium, fiber, and other nutrients may be beneficial in CKD, but serum potassium concentration should be monitored closely. A dose of fruits and vegetables that reduces potential renal acid load by 50% may reduce kidney injury, including albuminuria.10,12,13 Dietary protein reduction raises serum tCO2.18 However, this may lead to protein malnutrition and decreased muscle mass.76 Reduction of other acid-producing foods such as grains and cheeses can be considered.51
Summary
Metabolic acidosis is commonly treated to prevent adverse effects on bone, muscle, and kidney health. Results from interventional studies support this notion but are not definitive. There is biologic plausibility that acid-mediated organ injury occurs in a subset of patients with CKD with normal tCO2. These patients with subclinical metabolic acidosis may also benefit from alkali. Identifying these individuals is challenging and the dose to prescribe in this setting is unclear. Despite the apparent safety, there are potential risks of raising tCO2 using sodium-based alkali. Base therapy with fruits and vegetables is also a promising strategy to improve outcomes in CKD. Well designed, multicenter clinical trials investigating the long-term benefits and harms of alkali therapy in CKD are necessary.
Disclosures
None.
Acknowledgments
K.L.R. receives support from Career Development Award IK2 CX000537 from the US Department of Veterans Affairs Clinical Sciences Research and Development Service and National Institutes of Diabetes, Digestive, and Kidney Disease (1U01DK099933).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Van Slyke DD, Linder GC, Hiller A, Leiter L, McIntosh JF: The excretion of ammonia and titratable acid in nephritis. J Clin Invest 2: 255–288, 1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE: The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest 97: 1447–1453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May RC, Kelly RA, Mitch WE: Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J Clin Invest 79: 1099–1103, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemann J Jr., Litzow JR, Lennon EJ: The effects of chronic acid loads in normal man: Further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 45: 1608–1614, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemann J, Litzow JR, Lennon EJ: Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest 46: 1318–1328, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushinsky DA, Chabala JM, Gavrilov KL, Levi-Setti R: Effects of in vivo metabolic acidosis on midcortical bone ion composition. Am J Physiol 277: F813–F819, 1999 [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation: K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 35[6 Suppl 2]: S1–S140, 2000 [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[4 Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 9.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goraya N, Simoni J, Jo CH, Wesson DE: A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8: 371–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Goraya N, Simoni J, Jo CH, Wesson DE: Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Goraya N, Simoni J, Jo C, Wesson DE: Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int 81: 86–93, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, M’rad MB, Jacquot C, Houillier P, Stengel B, Fouqueray B; NephroTest Study Group : Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 20: 164–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raphael KL, Zhang Y, Ying J, Greene T: Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology (Carlton) 19: 648–654, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Raphael KL, Carroll DJ, Murray J, Greene T, Beddhu S: Urine ammonium predicts clinical outcomes in hypertensive kidney disease. J Am Soc Nephrol 28: 2483–2490, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gennari FJ, Hood VL, Greene T, Wang X, Levey AS: Effect of dietary protein intake on serum total CO2 concentration in chronic kidney disease: Modification of Diet in Renal Disease study findings. Clin J Am Soc Nephrol 1: 52–57, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Textor SC, Bravo EL, Fouad FM, Tarazi RC: Hyperkalemia in azotemic patients during angiotensin-converting enzyme inhibition and aldosterone reduction with captopril. Am J Med 73: 719–725, 1982 [DOI] [PubMed] [Google Scholar]
- 20.Tannen RL: Relationship of renal ammonia production and potassium homeostasis. Kidney Int 11: 453–465, 1977 [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 3:1–150, 2013 [Google Scholar]
- 22.Krieger NS, Sessler NE, Bushinsky DA: Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol 262: F442–F448, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH: Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol 8: 714–720, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D: Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: A prospective randomized single blind controlled trial. Ren Fail 28: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Starke A, Corsenca A, Kohler T, Knubben J, Kraenzlin M, Uebelhart D, Wüthrich RP, von Rechenberg B, Müller R, Ambühl PM: Correction of metabolic acidosis with potassium citrate in renal transplant patients and its effect on bone quality. Clin J Am Soc Nephrol 7: 1461–1472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein A, Moorhouse J, Iles-Smith H, Baker F, Johnstone J, James G, Troughton J, Bircher G, Walls J: Role of an improvement in acid-base status and nutrition in CAPD patients. Kidney Int 52: 1089–1095, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Graham KA, Reaich D, Channon SM, Downie S, Goodship TH: Correction of acidosis in hemodialysis decreases whole-body protein degradation. J Am Soc Nephrol 8: 632–637, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Graham KA, Reaich D, Channon SM, Downie S, Gilmour E, Passlick-Deetjen J, Goodship TH: Correction of acidosis in CAPD decreases whole body protein degradation. Kidney Int 49: 1396–1400, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre A, de Vernejoul MC, Gueris J, Goldfarb B, Graulet AM, Morieux C: Optimal correction of acidosis changes progression of dialysis osteodystrophy. Kidney Int 36: 1112–1118, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Weiner ID, Mitch WE, Sands JM: Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol 10: 1444–1458, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID: Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Vallet M, Metzger M, Haymann JP, Flamant M, Gauci C, Thervet E, Boffa JJ, Vrtovsnik F, Froissart M, Stengel B, Houillier P; NephroTest Cohort Study group : Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int 88: 137–145, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Clark EC, Nath KA, Hostetter MK, Hostetter TH: Role of ammonia in tubulointerstitial injury. Miner Electrolyte Metab 16: 315–321, 1990 [PubMed] [Google Scholar]
- 34.Nath KA, Hostetter MK, Hostetter TH: Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolins JP, Hostetter MK, Hostetter TH: Hypokalemic nephropathy in the rat. Role of ammonia in chronic tubular injury. J Clin Invest 79: 1447–1458, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita Y, Ikeguchi H, Nakamura J, Hotta N, Yuzawa Y, Matsuo S: Complement activation products in the urine from proteinuric patients. J Am Soc Nephrol 11: 700–707, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Kohan DE, Inscho EW, Wesson D, Pollock DM: Physiology of endothelin and the kidney. Compr Physiol 1: 883–919, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khanna A, Simoni J, Wesson DE: Endothelin-induced increased aldosterone activity mediates augmented distal nephron acidification as a result of dietary protein. J Am Soc Nephrol 16: 1929–1935, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Kohan DE, Barton M: Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 86: 896–904, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE: Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int 73: 192–199, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Wesson DE, Jo CH, Simoni J: Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR. Nephrol Dial Transplant 30: 762–770, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Wesson DE, Jo CH, Simoni J: Angiotensin II receptors mediate increased distal nephron acidification caused by acid retention. Kidney Int 82: 1184–1194, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Bento LM, Fagian MM, Vercesi AE, Gontijo JA: Effects of NH4Cl-induced systemic metabolic acidosis on kidney mitochondrial coupling and calcium transport in rats. Nephrol Dial Transplant 22: 2817–2823, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Pickering WP, Price SR, Bircher G, Marinovic AC, Mitch WE, Walls J: Nutrition in CAPD: Serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int 61: 1286–1292, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Schindler R: Causes and therapy of microinflammation in renal failure. Nephrol Dial Transplant 19[Suppl 5]: V34–V40, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Farwell WR, Taylor EN: Serum anion gap, bicarbonate and biomarkers of inflammation in healthy individuals in a national survey. CMAJ 182: 137–141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T: Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis 45: 275–280, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Alpern RJ, Sakhaee K: The clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 29: 291–302, 1997 [DOI] [PubMed] [Google Scholar]
- 49.DuBose TD, Jr.: Urine ammonium and preclinical acidosis in CKD. J Am Soc Nephrol 28: 2258–2260, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraut JA, Madias NE: Metabolic acidosis of CKD: An update. Am J Kidney Dis 67: 307–317, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Scialla JJ, Anderson CA: Dietary acid load: A novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis 20: 141–149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 24: 1232–1237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Wehbe E, Raina R, Simon JF, Srinivas TR, Jain A, Schreiber MJ Jr., Nally JV Jr.: Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol 6: 2395–2402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S: Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scialla JJ, Asplin J, Dobre M, Chang AR, Lash J, Hsu CY, Kallem RR, Hamm LL, Feldman HI, Chen J, Appel LJ, Anderson CA, Wolf M; Chronic Renal Insufficiency Cohort Study Investigators : Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int 91: 204–215, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scialla JJ, Appel LJ, Astor BC, Miller ER 3rd, Beddhu S, Woodward M, Parekh RS, Anderson CA; African American Study of Kidney Disease and Hypertension Study Group : Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int 82: 106–112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Berg E, Pasch A, Westendorp WH, Navis G, Brink EJ, Gans RO, van Goor H, Bakker SJ: Urinary sulfur metabolites associate with a favorable cardiovascular risk profile and survival benefit in renal transplant recipients. J Am Soc Nephrol 25: 1303–1312, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Born JC, Frenay AR, Bakker SJ, Pasch A, Hillebrands JL, Lambers Heerspink HJ, van Goor H: High urinary sulfate concentration is associated with reduced risk of renal disease progression in type 2 diabetes. Nitric Oxide 55-56: 18–24, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Jenkins AD, Dousa TP, Smith LH: Transport of citrate across renal brush border membrane: Effects of dietary acid and alkali loading. Am J Physiol 249: F590–F595, 1985 [DOI] [PubMed] [Google Scholar]
- 60.Frassetto L, Morris RC Jr., Sebastian A: Long-term persistence of the urine calcium-lowering effect of potassium bicarbonate in postmenopausal women. J Clin Endocrinol Metab 90: 831–834, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R: Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol 17: 3213–3222, 2006 [DOI] [PubMed] [Google Scholar]
- 62.He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, Dalton RN, Kaski JC, MacGregor GA: Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension 55: 681–688, 2010 [DOI] [PubMed] [Google Scholar]
- 63.U.S. National Library of Medicine: Alkali Therapy in Chronic Kidney Disease, 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT01452412. Accessed September 29, 2017
- 64.U.S. National Library of Medicine: Investigations of the Optimum Serum Bicarbonate Level in Renal Disease, 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT01574157. Accessed September 29, 2017
- 65.U.S. National Library of Medicine: The BASE Study: Bicarbonate Administration to Stabilize Estimated Glomerular Filtration Rate (eGFR) (BASE), 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT02521181. Accessed September 29, 2017
- 66.Fujii H, Iwase M, Ohkuma T, Ogata-Kaizu S, Ide H, Kikuchi Y, Idewaki Y, Joudai T, Hirakawa Y, Uchida K, Sasaki S, Nakamura U, Kitazono T: Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: The fukuoka diabetes registry. Nutr J 12: 159, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, Greene T, Beddhu S: High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81: 300–306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kotchen TA, Kotchen JM: Dietary sodium and blood pressure: Interactions with other nutrients. Am J Clin Nutr 65[Suppl]: 708S–711S, 1997 [DOI] [PubMed] [Google Scholar]
- 69.Luft FC, Steinberg H, Ganten U, Meyer D, Gless KH, Lang RE, Fineberg NS, Rascher W, Unger T, Ganten D: Effect of sodium chloride and sodium bicarbonate on blood pressure in stroke-prone spontaneously hypertensive rats. Clin Sci (Lond) 74: 577–585, 1988 [DOI] [PubMed] [Google Scholar]
- 70.Schmidlin O, Tanaka M, Sebastian A, Morris RC Jr.: Selective chloride loading is pressor in the stroke-prone spontaneously hypertensive rat despite hydrochlorothiazide-induced natriuresis. J Hypertens 28: 87–94, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidlin O, Tanaka M, Bollen AW, Yi SL, Morris RC Jr.: Chloride-dominant salt sensitivity in the stroke-prone spontaneously hypertensive rat. Hypertension 45: 867–873, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Dobre M, Yang W, Pan Q, Appel L, Bellovich K, Chen J, Feldman H, Fischer MJ, Ham LL, Hostetter T, Jaar BG, Kallem RR, Rosas SE, Scialla JJ, Wolf M, Rahman M; CRIC Study Investigators : Persistent high serum bicarbonate and the risk of heart failure in patients with chronic kidney disease (CKD): A report from the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Heart Assoc 4: 4, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schutte E, Lambers Heerspink HJ, Lutgers HL, Bakker SJ, Vart P, Wolffenbuttel BH, Umanath K, Lewis JB, de Zeeuw D, Gansevoort RT: Serum bicarbonate and kidney disease progression and cardiovascular outcome in patients with diabetic nephropathy: A post hoc analysis of the RENAAL (Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan) Study and IDNT (Irbesartan Diabetic Nephropathy Trial). Am J Kidney Dis 66: 450–458, 2015 [DOI] [PubMed] [Google Scholar]
- 74.de Solis AJ, González-Pacheco FR, Deudero JJ, Neria F, Albalate M, Petkov V, Susanibar L, Fernandez-Sanchez R, Calabia O, Ortiz A, Caramelo C: Alkalinization potentiates vascular calcium deposition in an uremic milieu. J Nephrol 22: 647–653, 2009 [PubMed] [Google Scholar]
- 75.Mendoza FJ, Lopez I, Montes de Oca A, Perez J, Rodriguez M, Aguilera-Tejero E: Metabolic acidosis inhibits soft tissue calcification in uremic rats. Kidney Int 73: 407–414, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Ikizler TA: Dietary protein restriction in CKD: The debate continues. Am J Kidney Dis 53: 189–191, 2009 [DOI] [PubMed] [Google Scholar]