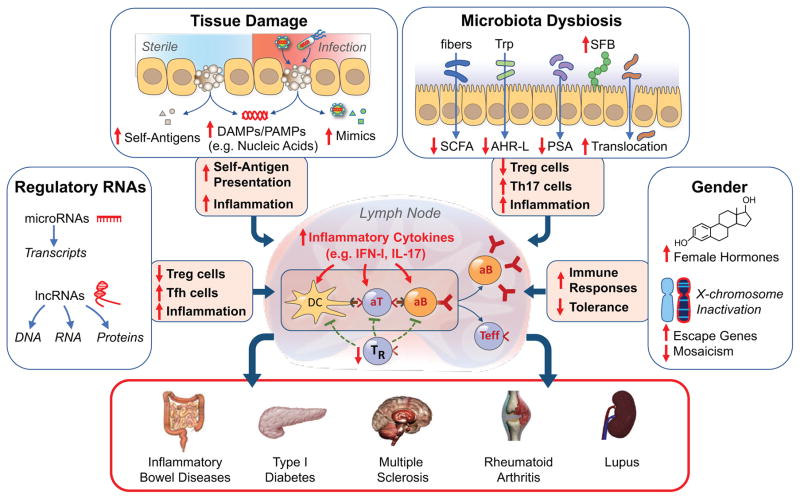
Figure 4. The multiple pathways to autoimmunity.
We posit that autoimmunity may result from disturbances in multiple processes acting singly or in combination. Tissue damage under sterile conditions or due to infections may lead to availability of nucleic acids and other damage- or pathogen-associated molecular patterns (DAMPs, PAMPs), presentation of self-antigens to non-tolerant lymphocytes, and induction of inflammatory responses. Microbiota dysbiosis may result in displacement of beneficial commensals, reductions of several anti-inflammatory factors (short chain fatty acids, SCFA; Aryl hydrocarbon receptor ligands, AHR-L; polysaccharide A, PSA), expansion of adherent bacteria (e.g. segmented filamentous bacteria, SFB in mice), damage of the mucosal/epithelial barrier, and translocation of bacteria and inflammatory products to mesenteric lymph nodes. These effects lead to engagement of TLRs and other innate sensors, production of inflammatory cytokines, reduction in Treg cells (TR), expansion of TH17 and other effector cells, and production of autoantibodies, resulting in organ-specific or systemic autoimmune diseases. Additional autoimmunity-contributing factors may include abnormalities in non-coding regulatory RNAs, gender-associated hormonal effects, and incomplete X chromosome inactivation. These processes require a predisposing genetic background for the pathogenic phenotype to be expressed.