Figure 7. Magnetic retention devices.
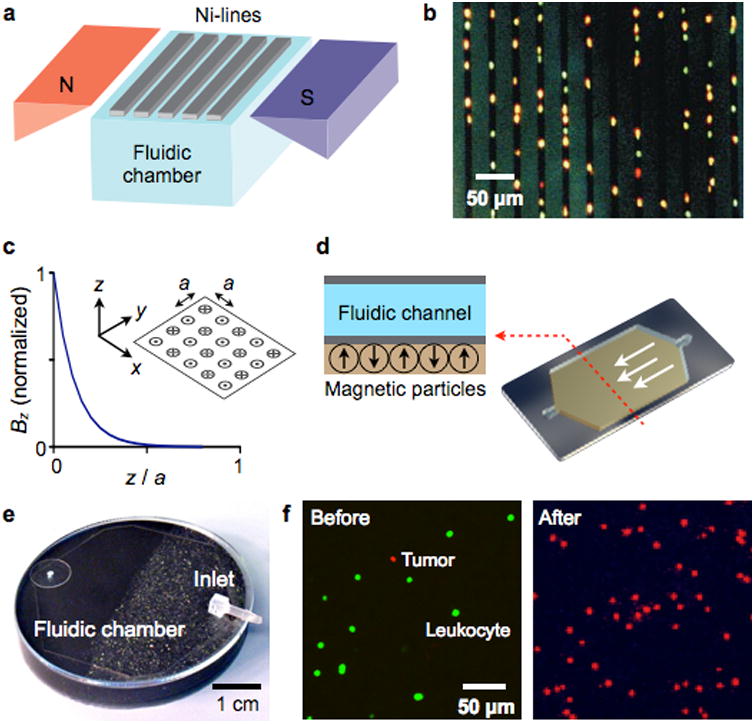
(a) Magnetic wire system. The separation chamber is optically transparent, but has ferromagnetic lines of nickel (Ni) deposited by lithography. The spacing between these lines is approximately the diameter of one white blood cell. When the chamber is placed between two angular-shaped magnets, the field gradients from the external magnets force the magnetically labeled cells upward to the top of the chamber. When the cells are in close proximity to Ni line, they are subjected to a high local gradient induced by the Ni lines. (b) The device in (a) was used to capture leukocytes that were labeled with CD45-specific MNPs. Because cells are aligned on the edge of Ni lines and counterstained with acridine orange, they can be easily observed by a microscope. (c) Alternating magnetic dipoles. This configuration creates magnetic fields that are tightly confined on the device surface. (d) When magnetic materials are allowed to self-assemble, the magnetic moments align into a similar pattern as in (c). The magnetic structure can easily cover the entire fluidic path to increase the throughput and the capturing efficiency. (e) A prototype device was implemented, that consisted of self-assembled layers of 125 μm grain (close to inlet) and 8 μm grain of NdFeB powder. (f) A suspension of leukocytes (stained green) and tumor cells were incubated with a mixture of magnetic beads conjugated with anti-CD45 antibodies and fluorescent antibodies against the tumor (anti-HER2/neu). The suspension was then flown through the magnetic device shown in (e). Fluorescence micrographs show the enrichment of tumor cells after the negative selection of leukocytes. The initial concentration of tumor cells to leukocytes was 1:10. Reproduced with permission from Ref. 90. Copyright 1999 Nature Publishing Group. Reproduced with permission from Ref. 97. Copyright 2011 RSC Publishing.
