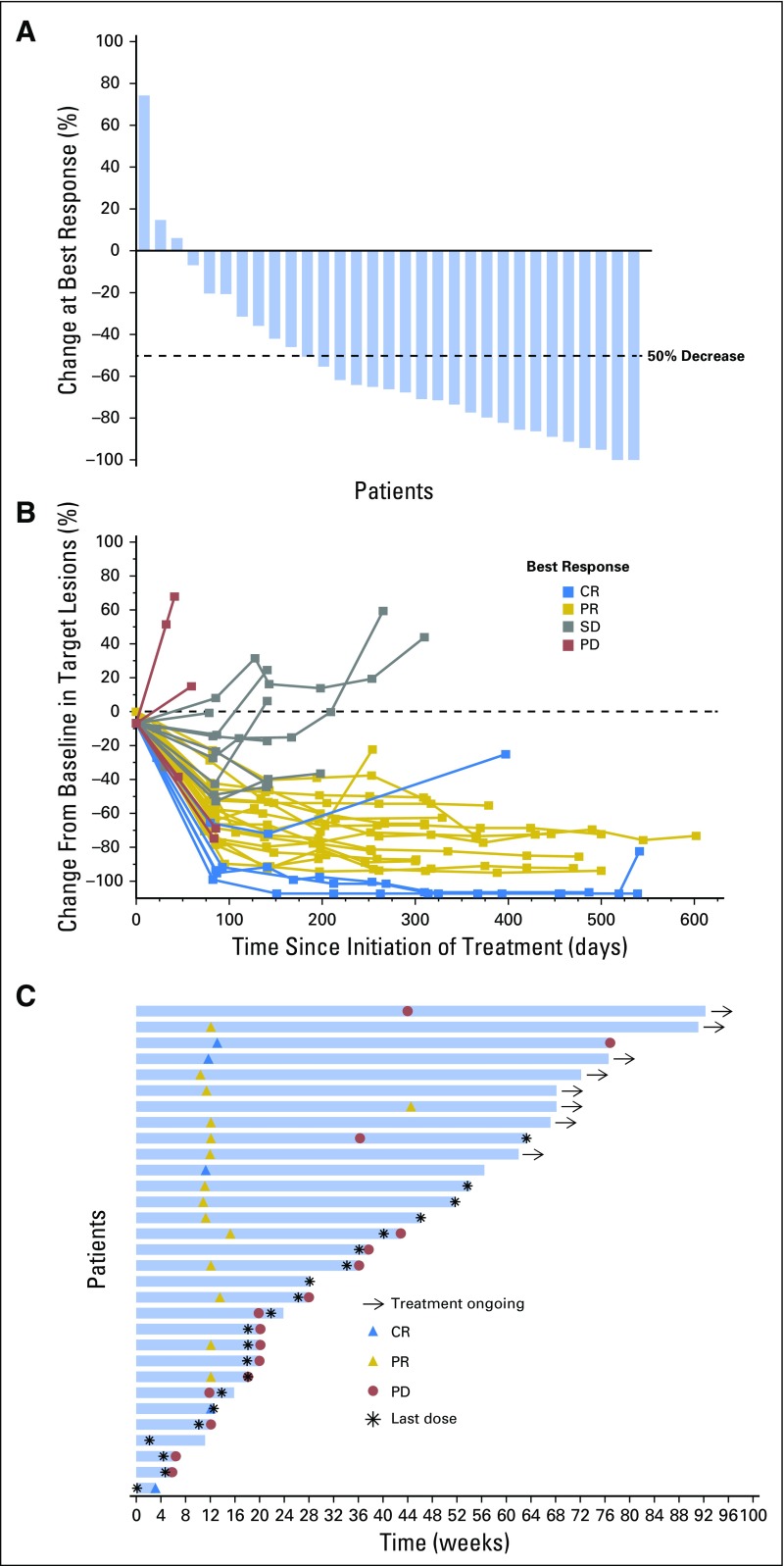
Fig 1.
Response to treatment. (A) Maximum percentage change from baseline in target lesions. (B) Change from baseline in target lesions. (C) Treatment exposure and response duration. Three patients had a formal response assessment before the protocol-required time point of 12 weeks. One patient only received one dose of pembrolizumab, discontinued treatment because of toxicity at 4 weeks, and had nonprotocol scans to assess response, which showed CR. The other two patients had nonprotocol scans to confirm the clinical impression of progressive disease before the 12-week time point. Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.