Abstract
Purpose
Everolimus improved median progression-free survival by 6.4 months in patients with advanced pancreatic neuroendocrine tumors (NET) compared with placebo in the RADIANT-3 study. Here, we present the final overall survival (OS) data and data on the impact of biomarkers on OS from the RADIANT-3 study.
Methods
Patients with advanced, progressive, low- or intermediate-grade pancreatic NET were randomly assigned to everolimus 10 mg/day (n = 207) or placebo (n = 203). Crossover from placebo to open-label everolimus was allowed on disease progression. Ongoing patients were unblinded after final progression-free survival analysis and could transition to open-label everolimus at the investigator’s discretion (extension phase). OS analysis was performed using a stratified log-rank test in the intent-to-treat population. The baseline levels of chromogranin A, neuron-specific enolase, and multiple soluble angiogenic biomarkers were determined and their impact on OS was explored.
Results
Of 410 patients who were enrolled between July 2007 and March 2014, 225 received open-label everolimus, including 172 patients (85%) randomly assigned initially to the placebo arm. Median OS was 44.0 months (95% CI, 35.6 to 51.8 months) for those randomly assigned to everolimus and 37.7 months (95% CI, 29.1 to 45.8 months) for those randomly assigned to placebo (hazard ratio, 0.94; 95% CI, 0.73 to 1.20; P = .30). Elevated baseline chromogranin A, neuron-specific enolase, placental growth factor, and soluble vascular endothelial growth factor receptor 1 levels were poor prognostic factors for OS. The most common adverse events included stomatitis, rash, and diarrhea.
Conclusion
Everolimus was associated with a median OS of 44 months in patients with advanced, progressive pancreatic NET, the longest OS reported in a phase III study for this population. Everolimus was associated with a survival benefit of 6.3 months, although this finding was not statistically significant. Crossover of patients likely confounded the OS results.
INTRODUCTION
Pancreatic neuroendocrine tumors (NET) account for approximately 1% of all cases of pancreatic cancers by incidence and 10% of all cases by prevalence.1-3 The incidence and prevalence of pancreatic NET are increasing.1-3
Patients with pancreatic NET, with the exception of those with insulinomas, are usually diagnosed at an advanced stage (approximately 64% of patients present with metastatic disease) and have poor prognosis.2 Therapeutic management of pancreatic NET depends on the degree of differentiation of the tumor (well v poorly differentiated), the stage at diagnosis, and the presence of symptoms caused by hypersecretion of hormones.4 Treatment options for patients with advanced, progressive pancreatic NET are limited.
Everolimus, an oral inhibitor of mammalian target of rapamycin, has shown antitumor activity in two phase II studies in patients with pancreatic NET who progressed after failure of chemotherapy.3,5 In the randomized, phase III RAD001 in Advanced Neuroendocrine Tumors, Third (RADIANT-3) trial, a statistically significant median progression-free survival (PFS) benefit of 6.4 months was achieved in patients with advanced pancreatic NET who received everolimus versus placebo.6
Here, we present the final overall survival (OS) data and update on safety information for the RADIANT-3 study population as predefined in the protocol, with 252 events observed as of March 2014. We also present the results of exploratory analysis of various tumor and angiogenic biomarkers and their impact on OS.
METHODS
Study Design and Participants
RADIANT-3 was a prospective, double-blind, randomized, placebo-controlled, multicenter, phase III study in which patients were randomly assigned to everolimus 10 mg/day or placebo, both in conjunction with best supportive care. Detailed inclusion and exclusion criteria and study methodology for the RADIANT-3 trial have been described previously.6 Adult patients (age, ≥ 18 years) with histologically confirmed, low- or intermediate-grade, advanced (unresectable or metastatic) pancreatic NET, who had radiologic disease progression documented within the 12 months before random assignment, were eligible. Additional eligibility criteria included the presence of measurable disease according to the Response Evaluation Criteria in Solid Tumors, version 1.0,7 WHO performance status ≤ 2, and adequate bone marrow, renal, and hepatic function. Patients who had received cytotoxic chemotherapy, immunotherapy, or radiotherapy within 4 weeks before random assignment, those who had received prior therapy with mammalian target of rapamycin inhibitors, and those who were receiving continuous treatment with corticosteroids or other immunosuppressive agents were excluded.
In the double-blind phase (core phase), patients continued to receive the treatment until disease progression, development of unacceptable adverse events (AEs), withdrawal of consent, or primary analysis (cutoff date, February 28, 2010). During this phase, crossover from the placebo arm to open-label everolimus was allowed on disease progression according to Response Evaluation Criteria in Solid Tumors, version 1.0.7 The data from the core phase were used for the primary efficacy end point of PFS and have been reported previously.6
After the primary analysis, all ongoing patients were unblinded on June 3, 2010, and were rolled over into an open-label extension phase in which the same safety assessments were performed as in the blinded treatment period. Treatment with open-label everolimus continued until disease progression on the basis of radiologic assessment. At this point, patients discontinued the study drug and were followed up for survival information on a monthly basis (with a 2-week window) until the required number of deaths for final OS analysis was observed (cutoff date, March 5, 2014). Patients not known to have died were censored for lost-to-follow-up if the time between their last contact date and the analysis cutoff date was > 44 days.
The study was conducted according to Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki. The institutional review board or independent ethics committee at each study site approved the protocol and all amendments. All patients provided written informed consent before random assignment.
Biomarker Analysis
Ten milliliters of whole blood was collected from all patients at screening (baseline). Plasma and serum samples were prepared at the trial sites and sent to a central laboratory for biomarker measurements. Chromogranin A (CgA) and neuron-specific enolase (NSE) concentrations were quantified in serum as described previously.8 Five soluble angiogenic biomarkers (placental growth factor [PIGF], soluble vascular endothelial growth factor receptor 1 and 2 [sVEGFR1 and 2], vascular endothelial growth factor A [VEGF-A], and basic fibroblast growth factor) were determined in plasma using commercially available enzyme-linked immunosorbent assay kits with Meso scale discovery platform Growth Factor Panels I and II assays at Tandem Laboratories/LabCorp (West Trenton, NJ).
Statistical Analyses
Final OS analysis was planned after approximately 250 events. OS was measured from the date of random assignment to the date of death due to any cause. For patients who did not die, survival was censored at the date of last contact. The OS analyses included all survival events among randomly assigned patients, regardless of whether they were observed during the core treatment period, the open-label treatment period, the post-treatment evaluations, or the survival follow-up period. Accounting for group-sequential design, the boundary for statistical significance at final analysis from the stratified one-sided log-rank test was 0.0249 (Appendix, online only).
Survival rates and median OS were estimated using the Kaplan-Meier method. Hazard ratios with 95% CIs were calculated for the OS using unadjusted Cox proportional hazards regression model.
An exploratory analysis for OS was performed using the Rank-Preserving Structural Failure Time (RPSFT) method. RPSFT provides a treatment effect estimate corrected for the confounding effect introduced by crossover.9 Hazard ratios and 95% CIs were calculated using Cox proportional hazard regression model to compare survival rates between everolimus and RPFST-corrected placebo.
Patients were divided into biomarker-elevated and -nonelevated subgroups on the basis of the baseline biomarker levels. Elevated baseline CgA and NSE were defined as > 2× upper limit of normal and > 1× upper limit of normal, respectively. For the angiogenic biomarkers, the median of distribution was used to define the threshold for elevated biomarker levels. Stratified Cox regression models were used to assess the prognostic values of the biomarkers. Hazard ratios with 95% CIs were reported between the biomarker subgroups irrespective of the treatment arms. The prognostic effects of the biomarkers were further investigated through a multivariate analysis.
AEs were coded using the Medical Dictionary for Regulatory Activities, version 16.1. The safety analyses included all AEs that occurred within 28 days after discontinuation of the study treatment. Key safety findings are presented for both the double-blind and the open-label phases.
Role of the Funding Source
The study was designed by the academic investigators and by representatives of the sponsor. Data were collected with the use of the sponsor’s data management systems and were analyzed by the sponsor’s statistical team, complying with study protocol and statistical analysis plan. Writing assistance funded by the sponsor was provided.
RESULTS
Patients and Treatment
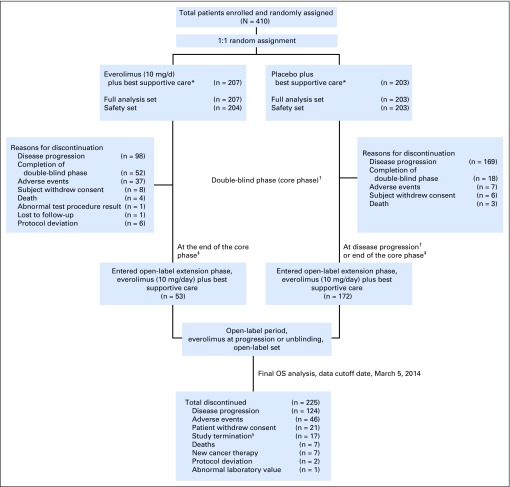
Of the 410 patients randomly assigned to everolimus (n = 207) or placebo (n = 203) between July 2007 to March 2014, a total of 225 patients eventually received open-label everolimus. These included 172 patients (85%) who crossed over from the placebo arm and 53 patients (26%) who were randomly assigned initially to the everolimus arm (Fig 1).
Fig 1.
Patient disposition. OS, overall survival. (*) Concurrent use of somatostatin analogs was allowed but not mandated. (†) At the time of progression during the double-blind phase, patients were unblinded and those randomly assigned to the placebo arm were allowed to cross over to open-label everolimus after assessment of benefit-risk by investigator on a case-by-case basis. (‡) All ongoing patients were unblinded at the end of the core phase (cutoff date, June 3, 2010) and were switched over to open-label everolimus. (§) At the time of study termination, 16 patients receiving everolimus were rolled over to study RAD001C2X01B (ClinicalTrials.gov identifier, NCT01789281) or commercial everolimus; one patient entered a compassionate use program in Canada.
Baseline demographic and clinical characteristics were well balanced between the treatment arms, particularly with respect to histologic status and prior receipt of radiotherapy, chemotherapy, or somatostatin analogue (SSA) therapy (Appendix Table A1, online only).6 During the double-blind period, use of concomitant SSAs was reported by 39.7% of patients in the everolimus arm and by 41.4% of patients in the placebo arm. In the open-label period, use of concomitant SSAs was reported in 45.3% of patients.
Median everolimus exposure was 38.9 weeks (range, 1.1 to 300.1 weeks) in patients who were randomly assigned initially to everolimus, and 44.1 weeks (range, 0.1 to 261.1 weeks) in those randomly assigned to placebo and switched subsequently to open-label everolimus.
Efficacy
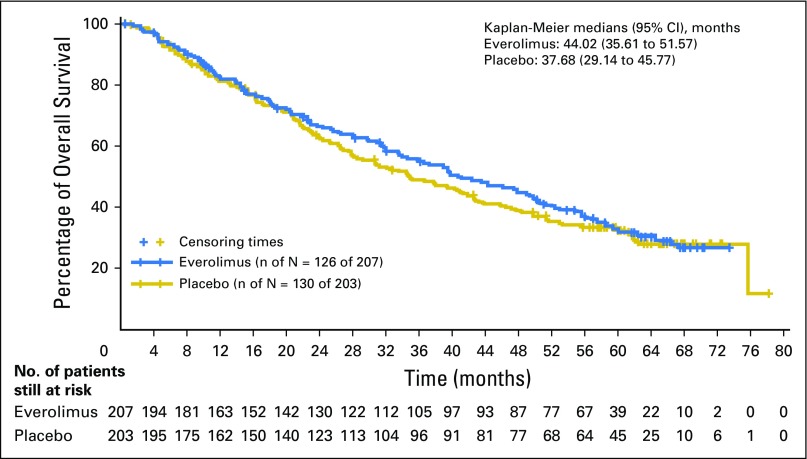
The median OS was 44.0 months (95% CI, 35.6 to 51.8 months) in the everolimus arm and 37.7 months (95% CI, 29.1 to 45.8 months) in the placebo arm, representing an improvement in median OS of 6.3 months over placebo (hazard ratio [HR], 0.94; 95% CI, 0.7 to 1.2). This difference did not achieve statistical significance (P = .30; Fig 2).
Fig 2.
Kaplan-Meier plot of overall survival (full analysis set).
Survival rates and duration at the 25th percentile are presented in the Appendix Table A2 (online only).
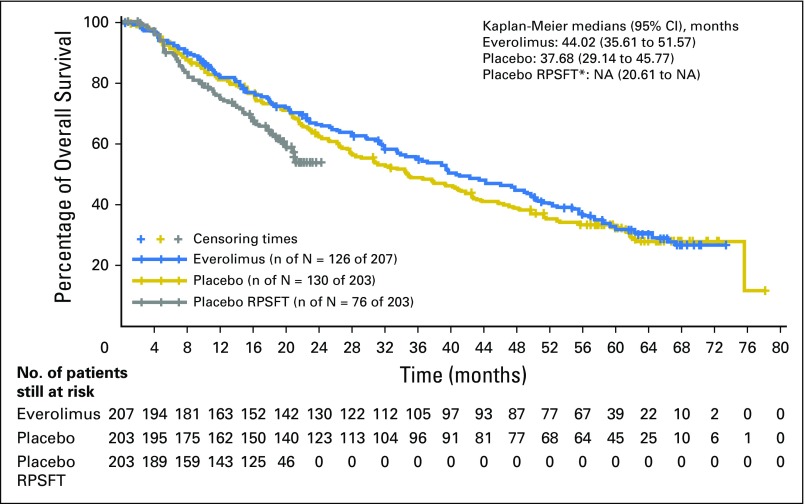
Using the RPSFT method to correct for crossover of patients from placebo to everolimus, 12- and 24-month survival rates were 82.6% and 67.7%, respectively, in the everolimus arm and 74.9% and ≤ 55.6%, respectively, in the RPSFT-corrected placebo arm (HR, 0.60; 95% CI, 0.09 to 3.95; Fig 3).
Fig 3.
Overall survival analysis by RPSFT (full analysis set). NA, not assessable; RPSFT, Rank-Preserving Structural Failure Time. (*) Reconstructed placebo data as if never treated with everolimus.
A total of 256 patients (62.4%) had died by the cutoff date of final survival analysis: 126 patients (60.9%) initially randomly assigned to the everolimus arm and 130 patients (64.0%) initially randomly assigned to the placebo arm. On the basis of investigator assessment, advanced or metastatic pancreatic NET was the primary cause of most of the deaths reported in this study (everolimus arm, 104 of 126 deaths [82.5%]; placebo arm, 111 of 130 deaths [85.4%]). Twenty-three of the 130 deaths in the placebo arm occurred before crossover of the treatment.
Overall, 154 patients were censored (including 109 patients lost to follow-up) from the OS analysis; of these, 81 were from the everolimus arm and 73 were from the placebo arm.
Biomarkers
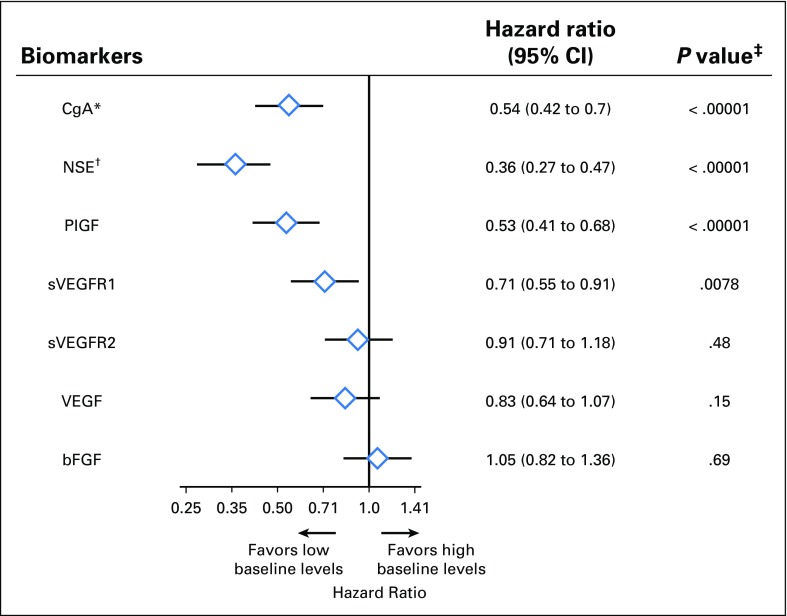
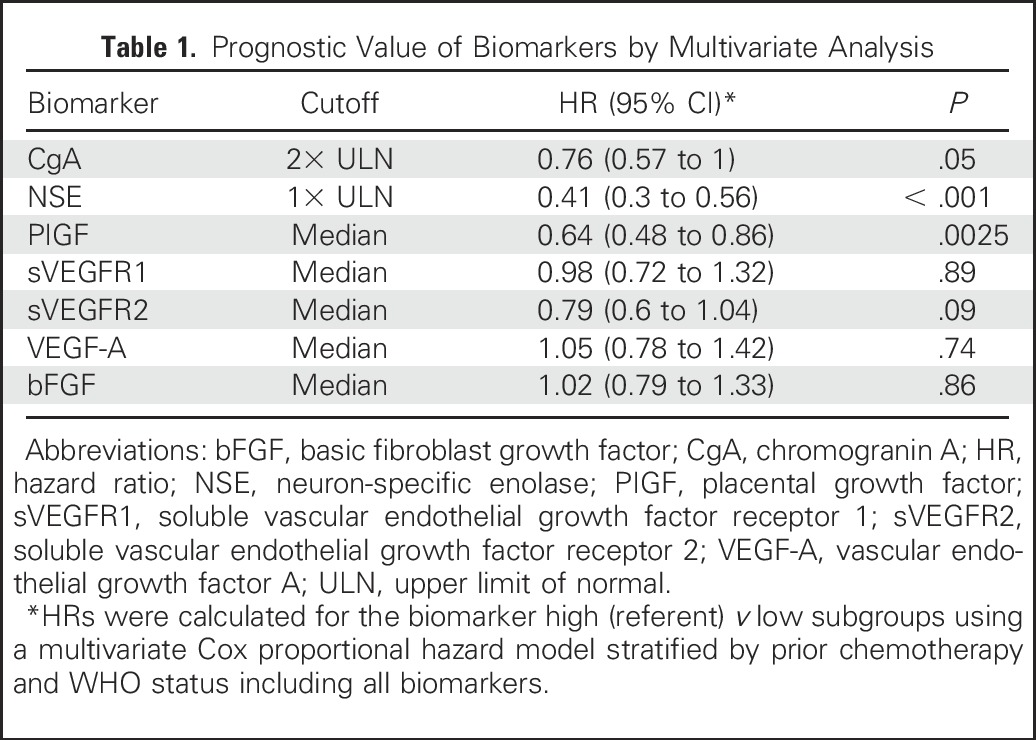
Evaluable baseline levels of seven soluble biomarkers were available from at least 95% of the intent-to-treat population (Appendix Table A3, online only). A prognostic effect was observed with baseline CgA, NSE, PIGF, and sVEGFR1 levels (HR, 0.54 for CgA, 0.36 for NSE, 0.53 for PIGF, and 0.71 for sVEGFR1; Fig 4). A multivariate analysis showed that the association of baseline levels of NSE and PIGF with survival holds when adjusting for the effect of other biomarkers (Table 1). The median OS rates of the biomarker subpopulations are given in the Appendix Table A4 (online only). No prognostic signal was detected for the rest of the angiogenic biomarkers.
Fig 4.
Prognostic effects of baseline chromogranin A (CgA), neuron-specific enolase (NSE), and angiogenic biomarkers. bFGF, basic fibroblast growth factor; PIGF, placental growth factor; SVEGFR, soluble VEGF receptor; VEGF, vascular endothelial growth factor. (*) Elevated baseline CgA was defined as > 2× upper limit of normal. (†) Elevated baseline NSE was defined as > 1× upper limit of normal. (‡) P values are nominal without adjustment for multiple testing. For all angiogenic biomarkers, the median of distribution was used to define the threshold for elevated biomarker levels.
Table 1.
Prognostic Value of Biomarkers by Multivariate Analysis
Safety
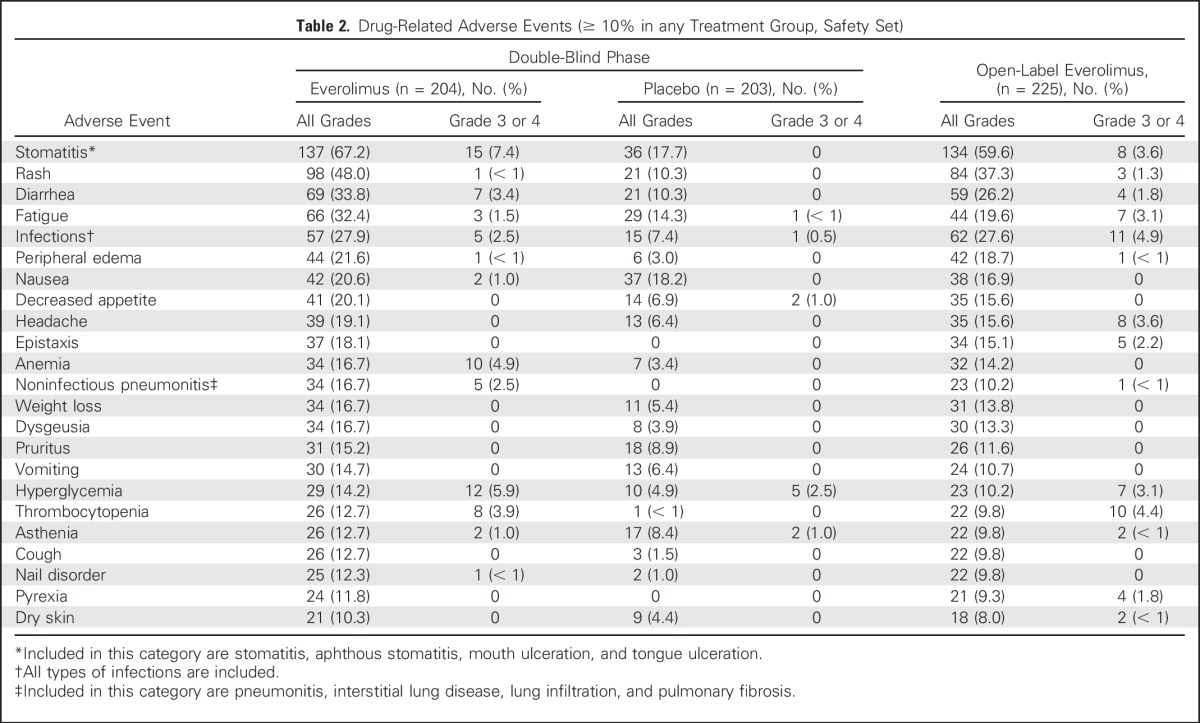
The safety findings were consistent with the previously reported safety profile of everolimus.6 No unexpected new safety findings were identified. In both the double-blind and the open-label phase, the most commonly reported AEs (≥ 20%) suspected to be drug related in the everolimus group were stomatitis, rash, diarrhea, fatigue, peripheral edema, nausea, and decreased appetite (Table 2). AEs reported in patients who received open-label everolimus were consistent with those observed during the double-blind phase.
Table 2.
Drug-Related Adverse Events (≥ 10% in any Treatment Group, Safety Set)
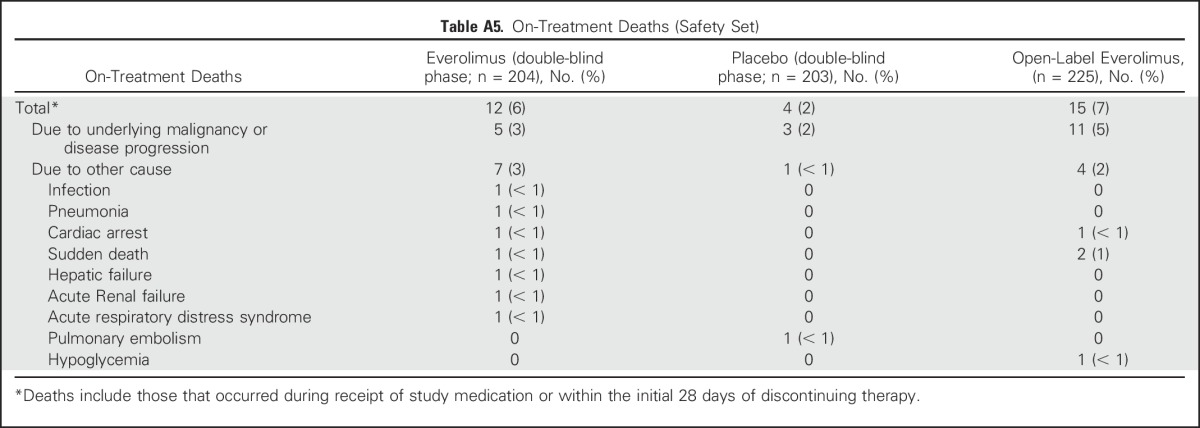
On-treatment deaths (ie, those occurring during receipt of study medication or within the initial 28 days of discontinuing therapy) were recorded for 16 patients in the double-blind phase; of these, 12 patients (5.9%) were in the everolimus arm and four (2.0%) were in the placebo arm. Of the 16 on-treatment deaths, eight (five everolimus and three placebo) were attributed to the underlying malignancy or progression thereof, and the remaining eight were attributed to other comorbidities. In the open-label phase, 15 on-treatment deaths occurred, of which 11 (4.9%) were attributed to the underlying malignancy (Appendix Table A5, online only). None of the on-treatment deaths in the double-blind and the open-label phase, with the exception of one in the everolimus arm caused by acute respiratory distress syndrome, were deemed to be related to the study drug, according to the investigators.
In the double-blind phase, serious AEs (SAEs) were reported more often in the everolimus arm (84 patients [41.2%] v 52 patients [25.6%] in the placebo arm). In the everolimus arm, 44 patients (21.6%) experienced SAEs that were suspected by the investigator to be drug related. The most commonly reported SAEs (≥ 2% incidence, irrespective of study drug relationship) in the everolimus arm were pyrexia, pneumonitis, anemia, abdominal pain, dyspnea, diarrhea, pulmonary embolism, asthenia, and dehydration. A total of 108 patients (48%) experienced SAEs during the open-label phase. Of these, 40 patients (17.8%) had SAEs that were suspected by the investigator to be drug related. Abdominal pain, vomiting, pneumonia, pyrexia, nausea, asthenia, GI hemorrhage, and cholangitis were the most frequently reported SAEs (≥ 2% incidence) in the open-label period (irrespective of causality).
The frequency of AEs leading to study drug discontinuation was more noticeable in the everolimus arm (21.1%) versus the placebo arm (5.9%) in the double-blind phase. The most frequent AEs leading to discontinuation in the everolimus group were pneumonitis (seven patients [3.4%]) and pyrexia (three patients [1.5%]). In the open-label extension phase, the rate of AEs leading to study drug discontinuation was similar to the one reported in the double-blind phase (53 patients [23.6%]; asthenia [four patients {1.8%}], hypoglycemia, pneumonia, and pneumonitis [each in three patients {1.3%}]) being the most common.
DISCUSSION
The final survival analysis of the pivotal phase III RADIANT-3 study showed a median OS of 44.0 months among patients with advanced, progressive pancreatic NET treated with everolimus. Although the prolongation in survival by 6.3 months with everolimus did not reach statistical significance, it is likely that crossover of the majority of patients from the placebo arm diluted the estimate of treatment effect. The RPSFT analysis adjusting for crossover bias supported a survival benefit with everolimus compared with the RPSFT-corrected placebo arm (82.6% v 74.9% and 67.7% v 55.6% at 12 months and 24 months, respectively).
Historically, median survival for patients with advanced pancreatic NET has been reported to be around 27 months.2 Streptozocin-based chemotherapy had been the standard of care for the treatment of pancreatic NET until the recent introduction of targeted therapies, such as everolimus and sunitinib. Randomized studies of streptozocin-based chemotherapy regimens in pancreatic NET have reported median OS in the range of 16 to 26 months.10,11 The availability of targeted therapies, everolimus and sunitinib, has changed the treatment paradigm of pancreatic NET and has likely improved survival in this patient population. A phase III study of sunitinib in pancreatic NET has reported a median survival of 38.6 months.12 Thus, a median OS of 44 months with everolimus from the randomized, placebo-controlled, phase III RADIANT-3 study establishes a new benchmark in the treatment landscape for patients with advanced, progressive pancreatic NET.
OS remains the most important clinically meaningful primary measurement of outcome for randomized trials in oncology. However, for clinical trials in rare tumors, such as pancreatic NET, the use of OS as the primary end point is particularly challenging because of extended postprogression survival, use of a range of salvage therapies after progression, and the crossover study design.13 The National Cancer Institute Neuroendocrine Tumor Clinical Trials Planning Meeting consensus report recommended PFS as a primary end point for clinical studies in the population of patients with NET, with OS being a secondary end point, as is the case with the RADIANT-3 study.14
The lack of a statistically significant survival benefit from everolimus in the RADIANT-3 study may have many reasons. The RADIANT-3 study permitted crossover of participants from the placebo arm to the everolimus arm on progression or at the time of unblinding. Crossover of the majority (approximately 85%) of the patients from the placebo arm likely diluted the estimation of true treatment effect on survival benefit. The results of the RPSFT analysis that corrected a potential crossover bias suggested a notable OS benefit with everolimus (HR, 0.6). This indeed confirms that crossover likely confounded the survival results. Although not intended to provide a formal proof-of-treatment effect, the RPSFT effect estimate was supportive of an everolimus survival benefit, albeit with wide CIs. The RADIANT-3 study was powered to detect an HR of 0.7 for OS. It is likely that the study did not reach the targeted HR because of the crossover design and longer postprogression survival than anticipated. It is recognized that the estimation of OS, as opposed to time to disease progression end points such as PFS, is sensitive to the confounding effects of poststudy treatment, which are generally subject to the discretion of the treating physician.15
Results from multiple studies have indicated the baseline CgA level as a prognostic factor for PFS in pancreatic NET and gastroenteropancreatic NET.8,16,17 NSE, however, is a less specific biomarker for NET, with only some preliminary association demonstrated with disease progression and survival. The results of this randomized, placebo-controlled, large phase III trial have confirmed that higher baseline levels of both CgA and NSE are poor prognostic factors of survival in patients with pancreatic NET. In this analysis, in fact, the NSE level seemed to be a stronger prognostic factor for OS than did CgA. This finding is consistent with our previous observation of their correlation with PFS in this trial.18 A potential clinical implication of this observation is to consider the baseline NSE level as a stratification factor in prospective randomized clinical trials in patients with pancreatic NET.
NET are highly vascularized and are capable of synthesizing VEGF to promote angiogenesis. Circulating proangiogenic biomarkers have been explored as predictors of efficacy for targeted agents such as everolimus, bevacizumab, sunitinib, and pazopanib and have a potential prognostic value in NET.18-20 The results of our prognostic analysis of angiogenic biomarkers for OS are consistent with a similar analysis for PFS in the RADIANT-3 study and other studies in patients with NET.18,21,22 A predictive signal for PFS was not identified for any of the biomarkers.18 Among the five angiogenic biomarkers, PIGF was identified as an independent prognostic factor on the basis of the multivariate analysis, suggesting that the circulating levels of the other molecules might have only a moderate effect on the disease progression. A meaningful correlation of OS with treatment of predictive signal search cannot be performed owing to crossover of a large number of patients from the placebo arm to the everolimus arm.
In conclusion, in the randomized, placebo-controlled, phase III RADIANT-3 study, everolimus demonstrated unprecedented median OS of 44.0 months in patients with advanced, progressive pancreatic NET. Although statistically not significant, the survival benefit of 6.3 months with everolimus is clinically meaningful. Survival was independent of baseline levels of angiogenic biomarkers or tumor biomarkers. A stronger OS advantage with everolimus after a correction for crossover effect confirms the presence of a likely confounding effect caused by crossover of the majority of patients from the placebo arm. The safety of everolimus was also consistent with previous experience, and no new safety findings were observed.
ACKNOWLEDGMENT
We thank Du Lam, who provided support in study conduct and interpretation of data. We also thank Rohit Kachhadiya and Bhavik Shah of Novartis Healthcare Pvt. for providing medical editorial assistance with this manuscript.
Appendix
METHODS
Overall Survival Estimation.
Progression-free survival (PFS) and overall survival (OS) were tested hierarchically. The OS analysis testing used one-sided group sequential log-rank tests with one interim analysis. If the test of primary end point of PFS was significant, an interim OS analysis was reported together with the PFS analysis (data cutoff date, February 28, 2010). The interim analysis of the OS test did not the cross prespecified boundary for statistical significance.6 At that time, the OS data were immature, with a total of 101 deaths reported in the study (51 in the everolimus arm and 50 in the placebo arm).
As per protocol, the final survival analysis was to be performed when approximately 250 events were reported. On the basis of the assumptions of a constant hazard ratio of 0.70, a total of 250 survival events would allow at least 80% power to demonstrate a 30% reduction of risk. The one-sided type I error of 0.025 was controlled by using a Lan-DeMets-O’Brien-Fleming error spending function. At the time of the final analysis, the nominal α level required for statistical significance was 0.0249.
RESULTS
Table A1.
Baseline Demographic and Disease Characteristics
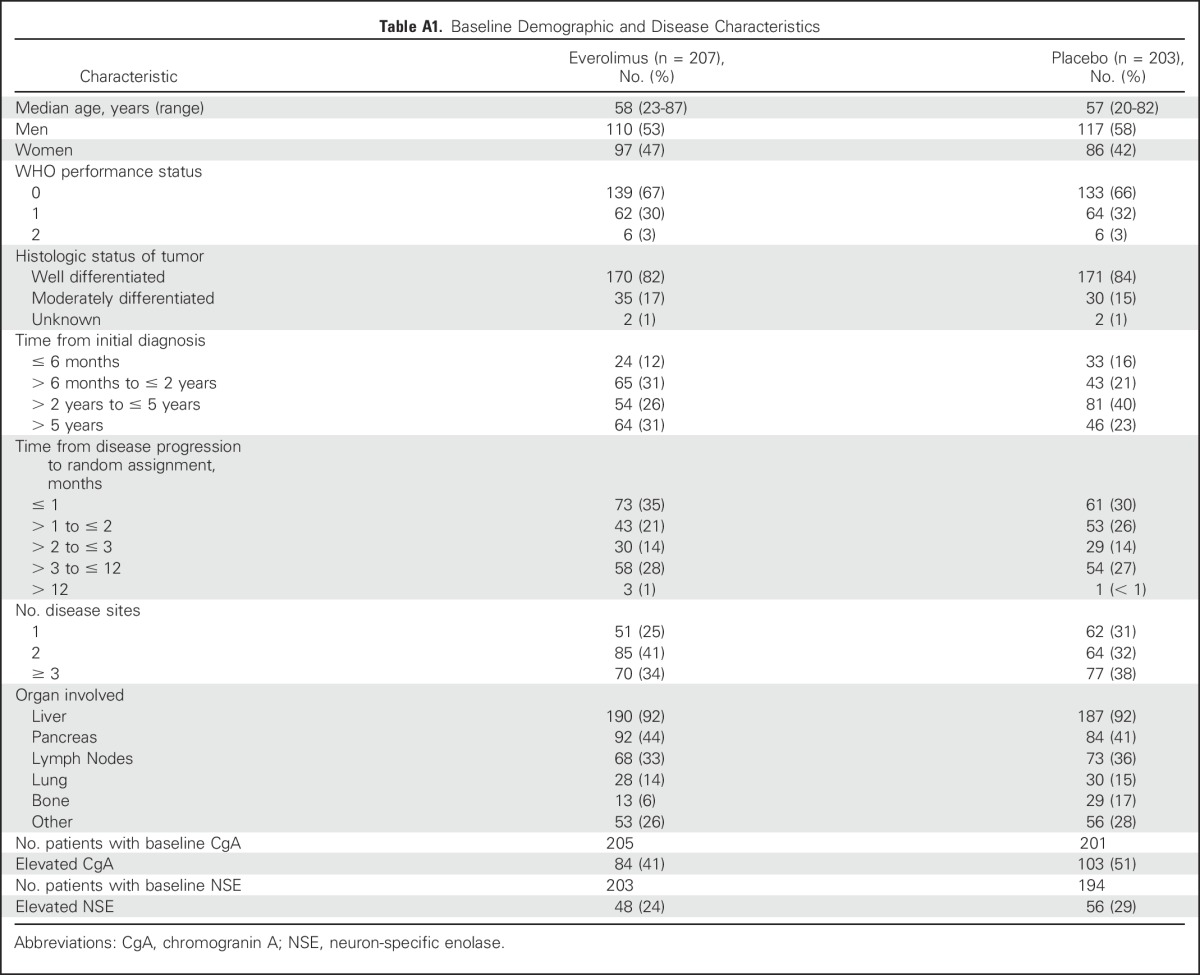
Table A2.
Yearly Kaplan-Meier Estimates
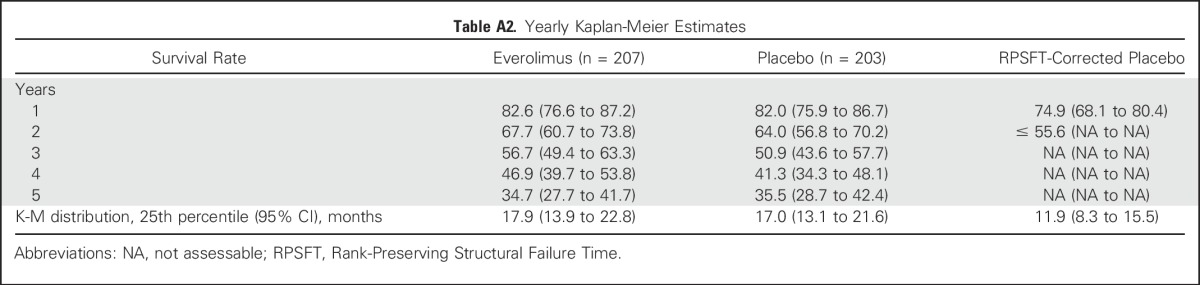
Table A3.
Median and Range of Baseline Biomarker Levels in Overall Biomarker Population and by Treatment Arm
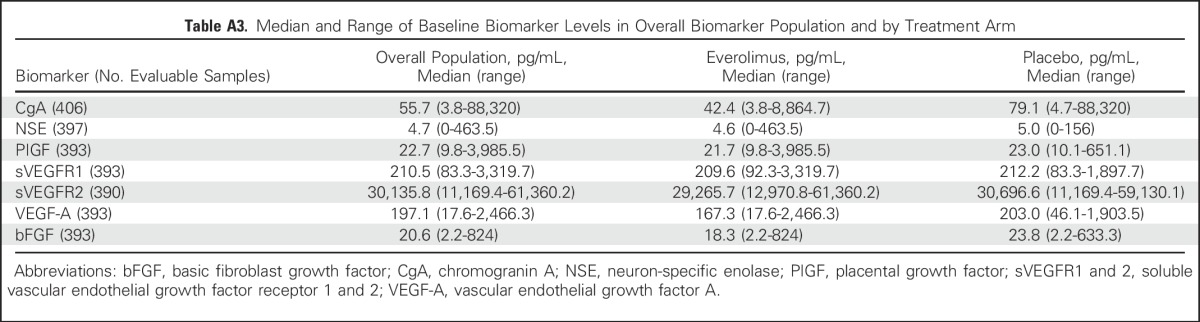
Table A4.
Median OS of Subgroups Defined by CgA (2× ULN as cutoff), NSE (1× ULN as cutoff), PIGF, sVEGFR1, sVEGFR2, VEGF, bFGF (median values as cutoff)
Table A5.
On-Treatment Deaths (Safety Set)
Footnotes
Supported by Novartis.
Presented in part at the Annual Meeting of the European Society for Medical Oncology, Madrid, Spain, September 26-31, 2014; the Annual Conference of the European Neuroendocrine Tumor Society, Barcelona, Spain, March 11-13, 2015; and the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00510068.
AUTHOR CONTRIBUTIONS
Conception and design: James C. Yao, Kjell Öberg
Provision of study materials or patients: James C. Yao, Marianne Pavel, Catherine Lombard-Bohas, Eric Van Cutsem, Jaume Capdevila, Elisabeth G.E. de Vries, Paola Tomassetti, Timothy Hobday, Rodney Pommier, Kjell Öberg
Collection and assembly of data: James C. Yao, Marianne Pavel, Catherine Lombard-Bohas, Eric Van Cutsem, Jaume Capdevila, Elisabeth G.E. de Vries, Paola Tomassetti, Timothy Hobday, Rodney Pommier, Kjell Öberg
Data analysis and interpretation: James C. Yao, Marianne Pavel, Maurizio Voi, Ulrike Brandt, Wei He, David Chen, Jaume Capdevila
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
James C. Yao
Consulting or Advisory Role: Novartis
Research Funding: Novartis (Inst)
Marianne Pavel
Consulting or Advisory Role: Novartis, Ipsen, Lexicon Pharmaceuticals
Research Funding: Novartis (Inst), Ipsen (Inst)
Catherine Lombard-Bohas
No relationship to disclose
Eric Van Cutsem
Research Funding: Amgen (Inst), Bayer AG (Inst), Boehringer Ingelheim (Inst), Eli Lilly (Inst), Merck Serono (Inst), Novartis (Inst), Roche (Inst), Sanofi (Inst), Celgene (Inst), Ipsen (Inst)
Maurizio Voi
Employment: Novartis, Mesoblast (I)
Stock or Other Ownership: Novartis
Ulrike Brandt
Employment: Novartis
Stock or Other Ownership: Novartis
Wei He
Employment: Novartis
David Chen
Employment: Novartis
Stock or Other Ownership: Novartis
Jaume Capdevila
No relationship to disclose
Elisabeth G.E. de Vries
Consulting or Advisory Role: Synthon (Inst), Medivation (Inst), Merck (Inst)
Research Funding: Novartis (Inst), Amgen (Inst), Genentech (Inst), SERVIER (Inst), Chugai Pharmaceutical (Inst), Synthon (Inst), AstraZeneca (Inst), Radius Health (Inst)
Travel, Accommodations, Expenses: Merck
Paola Tomassetti
No relationship to disclose
Timothy Hobday
No relationship to disclose
Rodney Pommier
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Travel, Accommodations, Expenses: Novartis
Kjell Öberg
Honoraria: Novartis, Ipsen
Consulting or Advisory Role: Novartis
Speakers’ Bureau: Novartis
REFERENCES
- 1.Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–3500. doi: 10.1245/s10434-007-9566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: A phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao J, Phan AT. Optimising therapeutic options for patients with advanced pancreatic neuroendocrine tumours. Eur Oncol Haematol. 2012;8:217–223. [Google Scholar]
- 5.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: Results of a phase II study. J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Yao JC, Pavel M, Phan AT, et al. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab. 2011;96:3741–3749. doi: 10.1210/jc.2011-0666. [DOI] [PubMed] [Google Scholar]
- 9.Korhonen P, Zuber E, Branson M, et al. Correcting overall survival for the impact of crossover via a rank-preserving structural failure time (RPSFT) model in the RECORD-1 trial of everolimus in metastatic renal-cell carcinoma. J Biopharm Stat. 2012;22:1258–1271. doi: 10.1080/10543406.2011.592233. [DOI] [PubMed] [Google Scholar]
- 10.Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303:1189–1194. doi: 10.1056/NEJM198011203032101. [DOI] [PubMed] [Google Scholar]
- 11.Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 12. Raymond E, Niccoli P, Castellano D, et al: Sunitinib (SU) in patients with advanced, progressive pancreatic neuroendocrine tumors (pNET): Final overall survival (OS) results from a phase III randomized study including adjustment for crossover. http://meetinglibrary.asco.org/content/158729-173.
- 13.Phan AT. Advances in the treatment of gastroenteropancreatic neuroendocrine tumors. Clin Adv Hematol Oncol. 2014;12(12, Suppl 19):3–5. [PubMed] [Google Scholar]
- 14.Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: Consensus report of the National Cancer Institute Neuroendocrine Tumor Clinical Trials Planning Meeting. J Clin Oncol. 2011;29:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Wang X, Law CH. Association between time to disease progression end points and overall survival in patients with neuroendocrine tumors. Gastrointest Cancer. 2014;4:103–113. [Google Scholar]
- 16.Chou WC, Chen JS, Hung YS, et al. Plasma chromogranin A levels predict survival and tumor response in patients with advanced gastroenteropancreatic neuroendocrine tumors. Anticancer Res. 2014;34:5661–5669. [PubMed] [Google Scholar]
- 17.Korse CM, Bonfrer JM, Aaronson NK, et al. Chromogranin A as an alternative to 5-hydroxyindoleacetic acid in the evaluation of symptoms during treatment of patients with neuroendocrine tumors. Neuroendocrinology. 2009;89:296–301. doi: 10.1159/000162876. [DOI] [PubMed] [Google Scholar]
- 18. Yao JC, Shah M, Panneerselvam A, et al: The VEGF pathway in patients with pancreatic neuroendocrine tumors: Efficacy of everolimus by baseline marker level, and prognostic and predictive effect analyses from RADIANT-3. Ann Oncol 23:ix376-ix382, 2012.
- 19.Mateo J, Heymach JV, Zurita AJ. Circulating biomarkers of response to sunitinib in gastroenteropancreatic neuroendocrine tumors: Current data and clinical outlook. Mol Diagn Ther. 2012;16:151–161. doi: 10.2165/11632590-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grande E, Capdevila J, Castellano D, et al. Pazopanib in pretreated advanced neuroendocrine tumors: A phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE) Ann Oncol. 2015;26:1987–1993. doi: 10.1093/annonc/mdv252. [DOI] [PubMed] [Google Scholar]
- 21.Pavel ME, Hassler G, Baum U, et al. Circulating levels of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin Endocrinol (Oxf) 2005;62:434–443. doi: 10.1111/j.1365-2265.2005.02238.x. [DOI] [PubMed] [Google Scholar]
- 22.Hilfenhaus G, Göhrig A, Pape UF, et al. Placental growth factor supports neuroendocrine tumor growth and predicts disease prognosis in patients. Endocr Relat Cancer. 2013;20:305–319. doi: 10.1530/ERC-12-0223. [DOI] [PubMed] [Google Scholar]