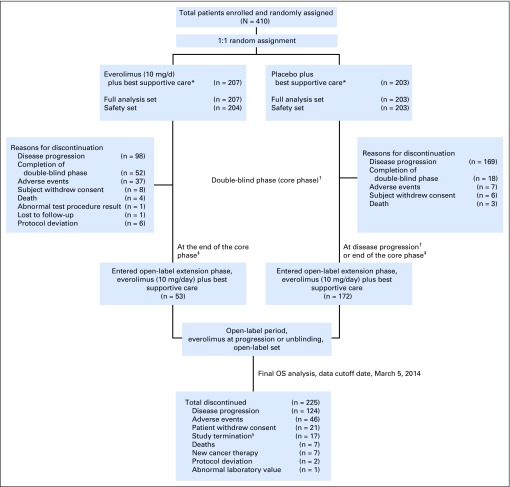
Fig 1.
Patient disposition. OS, overall survival. (*) Concurrent use of somatostatin analogs was allowed but not mandated. (†) At the time of progression during the double-blind phase, patients were unblinded and those randomly assigned to the placebo arm were allowed to cross over to open-label everolimus after assessment of benefit-risk by investigator on a case-by-case basis. (‡) All ongoing patients were unblinded at the end of the core phase (cutoff date, June 3, 2010) and were switched over to open-label everolimus. (§) At the time of study termination, 16 patients receiving everolimus were rolled over to study RAD001C2X01B (ClinicalTrials.gov identifier, NCT01789281) or commercial everolimus; one patient entered a compassionate use program in Canada.