Abstract
Urinary tract infection (UTI) is a broad term referring to an infection of the kidneys, ureters, bladder, and/or urethra. Because of its prevalence, frequent recurrence, and rising resistance to antibiotics, UTI has become a challenge in clinical practice. Autosomal-dominant polycystic kidney disease (ADPKD) is the most common monogenic disorder of the kidney and is characterized by the growth of fluid-filled cysts in both kidneys. Progressive cystic enlargement, inflammation, and interstitial fibrosis result in nephron loss with subsequent decline in kidney function. ADPKD patients frequently develop UTI; however, the cellular and molecular mechanisms responsible for the high UTI incidence in ADPKD patients remain virtually unaddressed. Emerging evidence suggests that α-intercalated cells (α-ICs) of the collecting ducts function in the innate immune defense against UTI. α-ICs inhibit bacterial growth by acidifying urine and secreting neutrophil gelatinase-associated lipocalin (NGAL) that chelates siderophore-containing iron. It is necessary to determine, therefore, if ADPKD patients with recurrent UTI have a reduced number and/or impaired function of α-ICs. Identification of the underlying cellular and molecular mechanisms may lead to the development of novel strategies to reduce UTI in ADPKD.
Keywords: urinary tract infection, autosomal-dominant polycystic kidney disease, intercalated cells, neutrophil gelatinase-associated lipocalin, iron sequester
autosomal-dominant polycystic kidney disease (ADPKD) is the most common monogenic kidney disorder, affecting 1 in 1,000 individuals worldwide (19). This hereditary disorder is characterized by the growth of fluid-filled cysts that replace normal renal tissue, resulting in gradual enlargement of both kidneys and progression to end-stage renal failure. Almost all forms of ADPKD are caused by genetic mutations in the PKD1 or PKD2 genes encoding polycystin 1 (PC1) and polycystin 2 (PC2), respectively (2). PC1 is a large glycoprotein with an extensive NH2-terminal extracellular region, 11 transmembrane domains, and a cytoplasmic COOH terminus with several phosphorylation signaling sites (reviewed in Refs. 33, 66). PC1 regulates PC2 channel activity and thus intracellular calcium homeostasis, and also plays a role in cell-cell/matrix interactions (21, 32, 68) and G protein-coupled signal transduction pathways (20, 45). High expression of PC1 in the basal membranes of ureteric bud epithelia suggests the involvement of PC1 during early development of the metanephric kidney (7, 25).
The available evidence indicates that PC2 functions as a calcium permeable cation channel involved in calcium signaling in renal epithelial cells. PC2 is also known as TRPP2 because of its high homology to other transient receptor protein channels. PC1 and PC2 interact and may be components of a multiprotein signaling complex involved in tubular morphogenesis (7, 49). Loss of either PC1 or PC2 function is thought to disrupt intracellular Ca2+ regulation, leading to the initiation of cyst formation (31, 69).
Urinary Tract Infections Are One of the Most Common Complications of ADPKD
UTI refers to an infection in any part of the urinary tract, including the kidneys, ureters, bladder, and urethra. UTI can be categorized into acute cystitis and acute pyelonephritis, which occur in the lower (bladder and urethra) and upper (kidneys and ureters) portions of the urinary tract, respectively. Over 8 million office visits and 1.7 million emergency room visits annually are attributed to UTI in the United States alone (23). The most recent data indicate that the medical costs for UTIs are more than 3.4 billion dollars in the United States in 2000 (34). Because of its prevalence, frequent recurrence, and rising resistance to antibiotics, UTI has evolved as a major challenge in clinical practice.
Approximately 30–50% of ADPKD patients suffer at least one UTI during their lifetime (15), indicating that UTI is a common feature of the disease. Patients with ADPKD after renal transplantation have a higher risk for native kidney infections than the general population (56). As in patients without ADPKD, UTI is usually incited by Gram-negative enteric organisms (53), which move up from the bladder. Other contributory factors include urinary tract instrumentation, nephrolithiasis, and vesicoureteral reflux (15). The most common causes of upper UTI in ADPKD are acute pyelonephritis and infected cysts (15). In both cases, patients typically present with flank pain and fever (15, 51). The presence of white cell casts is suggestive of acute pyelonephritis. An infected cyst may be present with a bland urine sediment and a sensitivity to pain at a specific area that relates to the location of the cyst infection (15). Moreover, both cyst and parenchymal infection may occur in any given subject. If UTI is suspected, a complete history and physical examination, blood and urine cultures with antimicrobial susceptibility testing, and a urinalysis can be performed to verify the diagnosis.
UTI Can Be Prevented
Clearly, there are multiple reasons for the development of UTIs in patients with ADPKD. However, a simple protective mechanism against the development of UTIs could result from increasing urine volume, in effect “flushing” the kidneys and minimizing stasis. There have been studies showing that an increase in urine volume may be protective against the development of UTIs, although the data is lacking (18, 35). Hence, drinking a large quantity of water every day may reduce the chances of getting UTI (28). Tolvaptan, a highly selective vasopressin V2 receptor antagonist has undergone phase 3 trials and is currently indicated for the treatment of ADPKD to minimize progression of disease leading to ESRD. Specifically, the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) 3:4 trial demonstrated a decrease in renal volume as well as an amelioration of worsening renal function (57). More recent analyses of these data documented a concomitant decrease in “kidney pain” [10.1 vs. 16.8% (P < 0.001)] (13). The authors concluded that one possible reason for the decreased pain could stem from a decrease in the rates of UTIs, with 15.3% of patients taking placebo developing UTI vs. 11.1% in the treatment group (13). These studies point to the aquaretic effects of Tolvaptan as a possible long-term protective effect against the development of UTIs. In addition, recent studies have shown that although polyuria is the most common side effect of Tolvaptan, it does not result in ureteral dilation, a metric for worsening renal function indicating that the diuretic effect in ADPKD patients does not result in disease progression (14). Clearly, general improvement in renal size and function from the use of Tolvaptan could lead to decreased rates of UTIs in ADPKD patients. However, as intercalated cells (see α-Intercalated Cells in the Renal Collect Duct System Protect Against UTI) do not have V2 receptors, the effects of Tolvaptan on intercalated cells and its specific effects on the development of UTI at this level needs further study.
Other steps to prevent UTI include 1) drinking cranberry juice, which is a good source of vitamin C that limits the growth of some bacteria by acidifying the urine (the same effect can be achieved by taking vitamin C supplements); 2) urinate in time or shortly after sex; 3) for females, wipe from front to back to prevent bacteria around the anus from going in the vagina or urethra, and do not use feminine hygiene sprays or scented douches; 4) take showers instead of tub baths; and 5) keep the genital area clean and dry. Cotton underwear and loose fitting clothes help keep the genital area dry. In contrast, tight clothes and nylon underwear trap moisture (28).
UTI Is Typically Treated with Antimicrobial Therapy
For all patients who have pronounced systemic symptoms such as flank pain, high fever, vomiting, and nausea due to cyst infection and/or acute pyelonephritis, intravenous ciprofloxacin or fluoroquinolone is recommended as the initiating antimicrobial therapy (15) as these drugs penetrate renal cysts. Intravenous therapy is adjusted based on the urine culture and discontinued once systemic symptoms disappear (15). An increasing problem in some centers is the development of fluoroquinolone resistance. Should this occur, cefotaxime or ampicillin plus gentamicin are alternate modalities for empiric therapy (15).
For patients with acute pyelonephritis and without evidence of cyst infection, a total course of therapy of at least 10 to 14 days is typical (15). Individuals with infected cysts can continue to receive oral fluoroquinolone for 4–6 wk (15). If patients do not have symptomatic improvement within 3 days or have persistent fevers for over 7 days, a spiral computerized tomography (CT) scan without contrast is usually recommended. The presence of a perinephric abscess or stones can be evaluated and the size of the infected cyst established by CT scan. Cysts over 5 cm in diameter do not usually respond to long-term parenteral antibiotic therapy (15, 16). Surgical or percutaneous drainage and nephrectomy are rarely required. Treatment of a perinephric abscess requires longer intravenous use of antibiotics that are effective toward the likely microorganisms, which are often Gram-negative (15).
Despite the frequency of UTI in ADPKD patients, the underlying cellular and molecular mechanisms that contribute to infection remain unknown. One possible cause is that urinary tracts of ADPKD patients are frequently obstructed by enlarged cysts and occasionally by kidney stones, which interfere with efficient clearance of microorganisms and promote formation of a “niche” for bacterial growth.
Most ADPKD Patients Have Normal Urine Acidification
Although acidified urine can inhibit bacterial growth (41), no studies have been reported to establish a direct link between UTI and a defect in urinary acidification. Moreover, whether ADPKD patients have an impaired capacity to acidify their urine is still controversial. Preuss et al. (48) first reported impaired urine acidification as an early manifestation of ADPKD. They found that 2 of 4 patients with ADPKD failed to decrease their urine pH to ≤5.3 in response to an acute administration of NH4CI. Such failure was observed only in 1 of 12 unaffected family members (48). Similarly, another independent study showed that 3 of 6 ADPKD patients with inulin clearances over 86 ml/min were incapable of acidifying their urine to pH 5.3 or below as compared with 3 of 10 normal family members (38). Inconsistent with these reports, two groups found a normal capacity of urine acidification in 7 ADPKD subjects (36, 58). ADPKD patients and age- and gender-matched controls were not significantly different in the urine pH following an acute NH4Cl challenge, although 1 of the ADPKD patients was unable to lower the urine pH to 5.3 or below (59). Based on these findings, it was thought that the majority of ADPKD patients with normal glomerular filtration rate can acidify their urine normally (59). Hence, UTI is less likely a result of impaired urine acidification, at least in some ADPKD patients.
α-Intercalated Cells in the Renal Collect Duct System Protect Against UTI
Organs with access to the outside environment contain specialized cells, such as clear cells in the epididymis, Paneth cells in the gastrointestinal tract, and intercalated cells in the frog skin. These specialized cells inhibit bacterial colonization by acidifying or alkalizing the adjacent fluid as well as by releasing antimicrobial peptides. α-Intercalated cells (α-ICs), which are critical for acid-base regulation by the kidney, also serve as specialized cells to protect the urinary tract from bacterial growth.
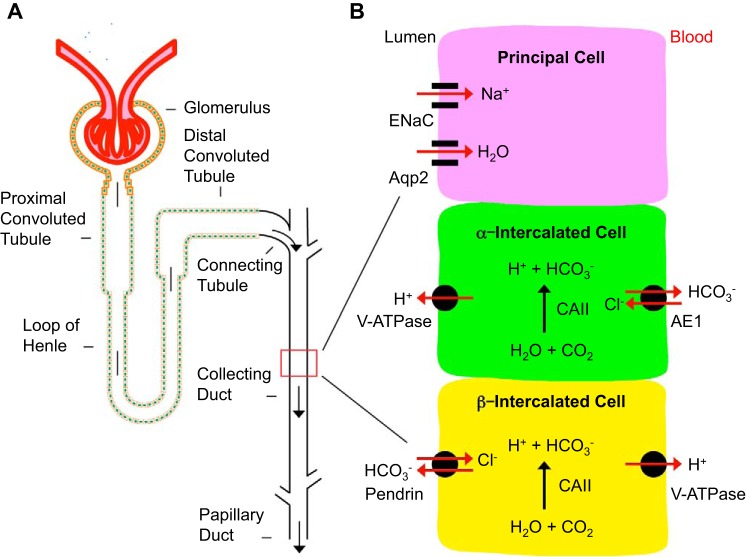
As shown in Fig. 1, the renal collecting duct in the mammalian kidney contains at least three structurally and functionally distinct cell types: principal cells (PCs), α-intercalated cells (α-ICs), and β-intercalated cells (β-ICs) (3). PCs play a key role in Na+ and water homeostasis. α-ICs and β-ICs regulate acid and bicarbonate secretion, respectively (1, 61). These cells are characterized by expressing a set of channels, transports, and exchangers including Aqp2 and ENaC in PCs and carbonic anhydrase type II (CAII) in all ICs (9, 10). The proton-pumping V-ATPase complex, which consists of at least 13 subunits including A, B1, and B2 is expressed in the plasma membranes of some epithelial cells including ICs in the kidney (11). The kidney variant of the band 3 Cl−/HCO3-exchanger (AE1) and the sodium-independent chloride/iodide transporter pendrin are markers of α-ICs and β-ICs, respectively (64). α-ICs express V-ATPase and other specific transporters for primary active H+ secretion into urinary tract. Urine pH is typically in the range of 4.5 to 6.3, which effectively attenuates bacterial growth (12). In addition to urine acidification, production and secretion of neutrophil gelatinase-associated lipocalin (NGAL) constitute a new mechanism to achieve bacteriostasis by α-ICs (41).
Fig. 1.
Cellular composition and function of renal collecting duct. A: diagram of a representative renal tubule. B: diagram showing the three types of cells in the collecting duct. Only representative markers are shown in each type of these cells to highlight their primary functions and to simplify the diagram.
NGAL Inhibits Bacterial Growth by Chelating Iron
Siderophores are small molecule compounds with high-affinity iron-chelating activity. They are among the effective soluble Fe3+ binding agents and utilized by pathogenic bacteria to acquire iron. NGAL belongs to the lipocalin family and binds enterochelin (Ent) (24), a siderophore synthesized by Gram-negative bacteria. Because of its high Fe3+ affinity, Ent can recruit Fe from transferrin to form an Ent:Fe3+ complex. In the presence of NGAL, an NGAL:Ent:Fe3+ complex is created facilitating degradation of Ent:Fe3+ and preventing accessibility of Fe3+ to bacteria in the urinary tract environment (5, 24). The NGAL:Ent:Fe3+ complex is highly stable, even at pH 4.0, suggesting that NGAL can sequester Ent:Fe3+ in most biological fluids, including acidified urine (39). Hence NGAL may have utility as a newly identified antimicrobial molecule that impairs bacterial growth by interacting with Ent:Fe3+ to diminish its fluid abundance (22). The critical role of NGAL in innate defense is evidenced by the phenotype of NGAL knockout mice. When challenged with systemic infection of Ent-expressing laboratory strains, these animals showed high infection rates and uncontrolled growth of uropathogenic Escherichia coli (6, 17, 41, 42).
The expression pattern of NGAL differs from that of the well-known antimicrobial peptides (40, 71) such as cathelicidins (hCAMP and mCRAMP), defensins (26, 60), uromodulin (54), and lactoferrin (60). These proteins are constitutively produced and mildly upregulated in response to infections. Normally, urinary NGAL is expressed only at low levels, suggesting a limited role at steady state. However, septic stimuli or other significant bacterial infections result in dramatic induction of NGAL production (4, 30, 46). Patients with tubular and tubulointerstitial damage, particularly when associated with infection, sepsis, or urosepsis, have intensely increased urinary NGAL levels.
α-ICs Are Apparently the Primary Source of Urinary NGAL
The source of urinary NGAL was discovered through the generation and characterization of NGAL-Luc2-mCherry bioluminescent reporter mice (43). These animals were created by inserting a double-fusion reporter gene encoding luciferase-2 and mCherry (Luc2-mC) at the start codon of the Lcn2 (NGAL) locus. The NGAL-Luc2-mCherry reporter faithfully and quantitatively recapitulated endogenous NGAL mRNA expression in several renal injury models. The reporter can also be continuously detected in real time to detect and follow kidney damage. Ischemia-reperfusion injury, systemic sepsis, and UTI rapidly induce reporter expression in the medulla. NGAL bioluminescence and urinary NGAL induction were identical in kinetics and amplitude of expression. In situ hybridization revealed NGAL expression in the thick ascending limb of Henle’s loop (TALH) and α-ICs, but not in β-ICs or PCs of the collecting duct after ischemia-reperfusion (43). Similar findings were made in several models of sepsis. In all of these scenarios, α-ICs prominently and consistently produced NGAL, whereas the TALH had variable expression (43).
To more directly address the physiological role of the ICs in UTI in vivo, Paragas et al. (41) developed kidney-specific Tcfcp2l1 conditional knockout mice by Cre-mediated recombination of floxed Tcfcp2l1 by using the Ksp-Cre transgene as the driver. Since Tcfcp2l1 encodes a transcription factor critical for IC differentiation, the mutant mice apparently lacked both α-IC and β-IC, as evidenced by the negative V-ATPase staining. V-ATPase is a well-known marker specific for ICs including α-IC, β-IC, and non-A/B type ICs. The distribution and density of segment-specific markers including PCs (Aqp2) and TALH (Tamm-Horsfall), serum creatinine, the urinary protein profiles, and the serum creatinine were normal in the Tcfcp2l1 conditional knockout mice (41). Consistent with the loss of α-ICs, urinary NGAL and urinary acidification were decreased in Tcfcp2l1fl/fl:Ksp-Cre mice, resulting in a significantly increased urinary bacterial load (41). These data suggest that α-ICs are crucial not only for regulating acid-base homeostasis but also for defense against UTI (41). In support of the concept that α-ICs serve as an overlooked component of the innate immune response, compromised ICs are responsible for the alkaline urine typical of distal renal tubular acidosis (dRTA), which is characterized by recurrent UTI, pyelonephritis, and kidney stones. It should be recognized, however, that the Tcfcp2l1 conditional knockout mice were created using the Ksp-Cre driver (55), which is active in developing nephrons, ureteric bud, mesonephric tubules, Wolffian duct, and Mullerian duct during embryogenesis, and its expression is detectable in renal tubules of the adult mouse kidney (55). The lack of the α-IC specificity of the driver may complicate data interpretation.
Do ADPKD Patients with UTI Have Fewer α-ICs?
Although renal cysts in ADPKD can arise from cells throughout the nephron (50), most cysts stain positive for markers of collecting duct cells in advanced ADPKD kidneys. However, indirect evidence exists suggesting that some ADPKD patients have reduced numbers of α-ICs. First, cells positive for carbonic anhydrase or AE1 were only occasionally found in renal cysts of patients with Meckel’s syndrome, indicating the rareness of α-ICs in cystic lesions (27). And second, immunofluorescence staining of Pkd1 nl/nl mouse renal cysts revealed the presence of α-ICs, as evidenced by the typical apical membrane staining of V-ATPase only in small cysts. By contrast, α-ICs were rarely seen in large cysts (70), presumably either because they are overgrown by other cells or because the α-ICs are subject to dedifferentiation or apoptosis during the progression of the disease. The reverse correlation between the α-IC abundance and the severity of the disease is also supported by the impact of cell-specific Pkd1 deletion on the severity of PKD. Disruption of Pkd1 in PCs resulted in more severe form of cystic kidney disease compared with knockout of Pkd1 in intercalated cells (50). It is unclear if enlarged cysts may have diminished ICs including α-ICs in these mouse models.
Given that UTI occurs in some but not all ADPKD patients, it is reasonable to speculate that ADPKD patients with UTI may have fewer α-ICs and lower NGAL expression. There are no reports, however, describing differences in the number of α-IC in ADPKD patients with or without UTI.
ADPKD Patients May Have Normal or Higher Levels of Urinary and Blood NGAL Levels
Studies on urinary and blood NGAL levels in ADPKD patients yielded inconsistent results. Both serum and urinary NGAL levels were significantly higher in ADPKD patients (n = 26) than in controls (8). Subjects with higher cystic growth had greater levels of NGAL in the serum and urine compared with others. It was suggested that tubular cells synthesize NGAL as a consequence of increased apoptosis following chronic damage or as a compensatory response, similar to that observed in acute stress conditions such as ischemia or renal toxicity and NGAL is implicated in cyst growth (8). Similarly, a study on the genotype-phenotype correlation revealed that plasma NGAL levels were higher in ADPKD patients already on renal replacement therapy (n = 18) compared with their affected relatives not on renal replacement therapy (n = 29), and plasma NGAL levels correlated closely with renal function (62). In contrast to these findings, a correlation between urinary NGAL and kidney function as measured by estimated glomerular filtration rate was not observed in ADPKD patients (n = 139) (47). Urinary NGAL was mildly and stably elevated in ADPKD patients (n = 107) enrolled in the Consortium for Radiologic Imaging for the Study of Polycystic Kidney Disease (CRISP), but it did not correlate with changes in total kidney volume or kidney function (44). In a prospective study involving ADPKD patients with confirmed mutations (PKD1 n = 33; PKD2 n = 17), no statistically significant correlation between higher plasma NGAL and ADPKD progression was found, and 18% of patients (9/50) had NGAL below limits of detection (60 pg/ml) (63).
The conflicting results may at least partially stem from the genetic heterogeneity, which renders ADPKD patients with or without UTI. Unfortunately, UTI was excluded from the analyses in all of the above studies. Classification of ADPKD based on the presence or absence of UTI may resolve the issue. Another contributory factor of the inconsistency is that NGAL is considered as a marker for both acute and chronic kidney injury and may not correlate with just the number of α-intercalated cells.
To directly investigate the effect of NGAL on PKD progression, three mouse strains were generated with different expression levels of NGAL within an established PKD model (Pkd1L3/L3): Pkd1L3/L3 (with endogenous NGAL), Pkd1L3/L3; NGALTg/Tg (with endogenous and overexpression of exogenous kidney-specific NGAL), and Pkd1L3/L3; NGAL−/− mice (with NGAL deficiency) (65). Elimination of endogenous NGAL did not significantly impact phenotypes, cystic progression, or survival of the PKD mice. Nevertheless, Pkd1L3/L3; NGALTg/Tg mice had a significantly longer lifespan, smaller and fewer renal cysts, and less interstitial fibrosis than the mice without or with endogenous NGAL. These changes were associated with reduced interstitial fibrosis, proliferation, and apoptosis (65).
Conclusion and Future Directions
UTI is one of the most common complications associated with ADPKD and a significant clinical challenge because of its prevalence, frequent recurrence, and increasing resistance to antibiotics. Nevertheless, little is known about the cellular and molecular basis of UTI in ADPKD. Recent studies suggest that α-ICs in the collecting duct play an important role in the defense of UTI by synthesizing and secreting NGAL. NGAL not only acidifies urine but also sequesters Fe3+ to achieve a bacteriostatic effect. These new discoveries may offer a clue as to how UTI is linked to ADPKD at the cellular and molecular level. Future studies should address two important questions: 1) Are α-ICs truly the primary source of urinary NGAL? and 2) Do ADPKD kidneys with UTI have decreased numbers and/or debilitated function of α-ICs to secrete NGAL into cyst fluid and urine than those without UTI?
Whereas the second question is relatively straight forward, conclusively addressing the first question is problematic. As discussed above, utility of Tcfcp2lfl/fl Ksp-Cre mice may be compromised since the Ksp-Cre driver does not restrict Cre expression exclusively to α-ICs. Although the V-ATPase B1-Cre may be considered as an alternative, it is also not specific for α-ICs as Cre expression was evident in all ICs (α-IC, β-IC, and non-A/B cells) within the collecting duct and most cells of the connecting segment. About 50% of the PCs of the connecting segment also expressed Cre (37). The anion exchange protein 1 (AE1, known as band-3 anion transport protein as well) promoter is apparently an attractive potential Cre driver because AE1 is a well-established α-IC marker (52). However, SLC4A1, which encodes AE1, generates a different isoform of AE1 in erythrocytes (29). If AE1 promoter activates Cre expression in the progenitor cells of erythrocytes, deletion of genes of interest on Cre-mediated recombination may impact erythropoiesis and/or immune cell function, complicating data analysis and interpretation.
Most ICs (including α-IC and β-IC) as well as the PCs are derived from Aqp2+ progenitor cells (67). Their common origin adds another layer of concern. With the loss of histone H3 K79 dimethylation as a tracing marker, we reported that not only the PCs but also the majority of ICs including α-IC and β-IC arise from a new pool of progenitor cells expressing Aqp2 in Dot1lAC mice (67). Using the red fluorescence protein variant (tdTomato) as the lineage tracing marker, we verified that Aqp2+ progenitor cells differentiate into ICs naturally (i.e., without Dot1l inactivation) and contribute to renal intertubular connection by giving rise to transitional cells at the junction between distal convoluted tubule and connecting tubule (unpublished data). Hence, ablation of any genes crucial for specification of the principal and intercalated cells may cause abnormal development of the collecting duct system and impairment in the defense of UTIs.
GRANTS
Support for this study was provided by National Institute of Diabetes and Digestive and Kidney Diseases Grants 2R01 DK-080236 06A1 and R21 DK-70834 (to W. Zhang); and R01 DK-081579 (to D. Wallace).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.G., L.Z., Y.Z., D.P.W., R.I.L.-S., P.J.H., and W.Z. edited and revised manuscript; C.G., L.Z., Y.Z., D.P.W., R.I.L.-S., P.J.H., and W.Z. approved final version of manuscript; D.P.W. and W.Z. conceived and designed research; D.P.W. and W.Z. analyzed data; D.P.W. and W.Z. interpreted results of experiments; P.J.H. and W.Z. prepared figures; W.Z. performed experiments; W.Z. drafted manuscript.
REFERENCES
- 1.Al-Awqati Q. Plasticity in epithelial polarity of renal intercalated cells: targeting of the H+-ATPase and band 3. Am J Physiol Cell Physiol 270: C1571–C1580, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Audrézet MP, Corbiere C, Lebbah S, Morinière V, Broux F, Louillet F, Fischbach M, Zaloszyc A, Cloarec S, Merieau E, Baudouin V, Deschênes G, Roussey G, Maestri S, Visconti C, Boyer O, Abel C, Lahoche A, Randrianaivo H, Bessenay L, Mekahli D, Ouertani I, Decramer S, Ryckenwaert A, Cornec-Le Gall E, Salomon R, Ferec C, Heidet L. Comprehensive PKD1 and PKD2 mutation analysis in prenatal autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 722–729, 2016. doi: 10.1681/ASN.2014101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnis C, Marshansky V, Breton S, Brown D. Remodeling the cellular profile of collecting ducts by chronic carbonic anhydrase inhibition. Am J Physiol Renal Physiol 280: F437–F448, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, D’amico G, Goldsmith D, Devarajan P, Bellomo R. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med 36: 452–461, 2010. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 5.Barasch J, Mori K. Cell biology: iron thievery. Nature 432: 811–813, 2004. doi: 10.1038/432811a. [DOI] [PubMed] [Google Scholar]
- 6.Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA 103: 1834–1839, 2006. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhaskar LV, Elumalai R, Periasamy S. Pathways, perspectives and pursuits in polycystic kidney disease. J Nephropharmacol 5: 41–48, 2015. [PMC free article] [PubMed] [Google Scholar]
- 8.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol 27: 373–378, 2007. doi: 10.1159/000103912. [DOI] [PubMed] [Google Scholar]
- 9.Breton S, Alper SL, Gluck SL, Sly WS, Barker JE, Brown D. Depletion of intercalated cells from collecting ducts of carbonic anhydrase II-deficient (CAR2 null) mice. Am J Physiol Renal Fluid Electrolyte Physiol 269: F761–F774, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Brown D, Kumpulainen T, Roth J, Orci L. Immunohistochemical localization of carbonic anhydrase in postnatal and adult rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 245: F110–F118, 1983. [DOI] [PubMed] [Google Scholar]
- 11.Brown D, Paunescu TG, Breton S, Marshansky V. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol 212: 1762–1772, 2009. doi: 10.1242/jeb.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlsson S, Wiklund NP, Engstrand L, Weitzberg E, Lundberg JO. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide 5: 580–586, 2001. doi: 10.1006/niox.2001.0371. [DOI] [PubMed] [Google Scholar]
- 13.Casteleijn NF, Blais JD, Chapman AB, Czerwiec FS, Devuyst O, Higashihara E, Leliveld AM, Ouyang J, Perrone RD, Torres VE, Gansevoort RT; TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes) 3:4 Trial Investigators . Tolvaptan and kidney pain in patients with autosomal dominant polycystic kidney disease: secondary analysis from a randomized controlled trial. Am J Kidney Dis 69: 210–219, 2017. doi: 10.1053/j.ajkd.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casteleijn NF, Messchendorp AL, Bae KT, Higashihara E, Kappert P, Torres V, Meijer E, Leliveld AM. Polyuria due to vasopressin V2 receptor antagonism is not associated with increased ureter diameter in ADPKD patients. Clin Exp Nephrol 21: 375–382, 2017. doi: 10.1007/s10157-016-1297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman AB, Rahbari-Oskoui FF, Bennett WM. Urinary tract infection in autosomal dominant polycystic kidney disease. http://www.uptodate.com/contents/urinary-tract-infection-in-autosomal-dominant-polycystic-kidney-disease [24 Jul 2017].
- 16.Chapman AB, Thickman D, Gabow PA. Percutaneous cyst puncture in the treatment of cyst infection in autosomal dominant polycystic kidney disease. Am J Kidney Dis 16: 252–255, 1990. doi: 10.1016/S0272-6386(12)81025-0. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Zhang W. Kidney α-intercalated cells, NGAL and urinary tract infection. Austin J Nephrol Hypertens 1: 1017, 2014. [PMC free article] [PubMed] [Google Scholar]
- 18.Choi HY, Park HC, Ha SK. High water intake and progression of chronic kidney diseases. Electrolyte Blood Press 13: 46–51, 2015. doi: 10.5049/EBP.2015.13.2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Miranda Henriques MS, de Morais Villar EJ. The liver and polycystic kidney disease. In: Polycystic Kidney Disease, edited by Li X. Brisbane, Australia: Codon Publications, 2015, chapt. 17. doi: 10.15586/codon.pkd.2015.ch17. [DOI] [PubMed] [Google Scholar]
- 20.Delmas P, Nomura H, Li X, Lakkis M, Luo Y, Segal Y, Fernández-Fernández JM, Harris P, Frischauf AM, Brown DA, Zhou J. Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J Biol Chem 277: 11276–11283, 2002. doi: 10.1074/jbc.M110483200. [DOI] [PubMed] [Google Scholar]
- 21.Drummond IA. Polycystins, focal adhesions and extracellular matrix interactions. Biochim Biophys Acta 1812: 1322–1326, 2011. doi: 10.1016/j.bbadis.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432: 917–921, 2004. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 23.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon 49: 53–70, 2003. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 24.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 10: 1033–1043, 2002. doi: 10.1016/S1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 25.Griffin MD, O’Sullivan DA, Torres VE, Grande JP, Kanwar YS, Kumar R. Expression of polycystin in mouse metanephros and extra-metanephric tissues. Kidney Int 52: 1196–1205, 1997. doi: 10.1038/ki.1997.444. [DOI] [PubMed] [Google Scholar]
- 26.Hiratsuka T, Nakazato M, Ihi T, Minematsu T, Chino N, Nakanishi T, Shimizu A, Kangawa K, Matsukura S. Structural analysis of human β-defensin-1 and its significance in urinary tract infection. Nephron 85: 34–40, 2000. doi: 10.1159/000045627. [DOI] [PubMed] [Google Scholar]
- 27.Holthöfer H, Kumpulainen T, Rapola J. Polycystic disease of the kidney. Evaluation and classification based on nephron segment and cell-type specific markers. Lab Invest 62: 363–369, 1990. [PubMed] [Google Scholar]
- 28.The Johns Hopkins University Urinary tract infections or UTIs. http://www.hopkinsmedicine.org/healthlibrary/conditions/kidney_and_urinary_system_disorders/urinary_tract_infections_utis_85,P01497 [24 Jul 2017].
- 29.Kollert-Jöns A, Wagner S, Hübner S, Appelhans H, Drenckhahn D. Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol Renal Fluid Electrolyte Physiol 265: F813–F821, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Kümpers P, Hafer C, Lukasz A, Lichtinghagen R, Brand K, Fliser D, Faulhaber-Walter R, Kielstein JT. Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Crit Care 14: R9, 2010. doi: 10.1186/cc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo IY, DesRochers TM, Kimmerling EP, Nguyen L, Ehrlich BE, Kaplan DL. Cyst formation following disruption of intracellular calcium signaling. Proc Natl Acad Sci USA 111: 14283–14288, 2014. doi: 10.1073/pnas.1412323111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee K, Boctor S, Barisoni LM, Gusella GL. Inactivation of integrin-β1 prevents the development of polycystic kidney disease after the loss of polycystin-1. J Am Soc Nephrol 26: 888–895, 2015. doi: 10.1681/ASN.2013111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X. Phosphorylation, protein kinases and ADPKD. Biochim Biophys Acta 1812: 1219–1224, 2011. doi: 10.1016/j.bbadis.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Litwin MS, Saigal CS, Yano EM, Avila C, Geschwind SA, Hanley JM, Joyce GF, Madison R, Pace J, Polich SM, Wang M; Urologic Diseases in America Project . Urologic Diseases in America Project: analytical methods and principal findings. J Urol 173: 933–937, 2005. doi: 10.1097/01.ju.0000152365.43125.3b. [DOI] [PubMed] [Google Scholar]
- 35.Lotan Y, Daudon M, Bruyère F, Talaska G, Strippoli G, Johnson RJ, Tack I. Impact of fluid intake in the prevention of urinary system diseases: a brief review. Curr Opin Nephrol Hypertens 22, Suppl 1: S1–S10, 2013. doi: 10.1097/MNH.0b013e328360a268. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Maldana M. Functional aspects: electrolyte and uric acid excretion, In: Problems in Diagnosis and Management of Polycystic Kidney Disease, edited by Grantham JJ, Gardner KD; PKR Foundation . Kansas City, MO: Intercollegiate Press, 1985, p. 70–80. [Google Scholar]
- 37.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, Nelson RD. The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009. doi: 10.1038/ki.2008.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milutinovic J, Agodoa LC, Cutler RE, Striker GE. Autosomal dominant polycystic kidney disease. Early diagnosis and consideration of pathogenesis. Am J Clin Pathol 73: 740–747, 1980. doi: 10.1093/ajcp/73.6.740. [DOI] [PubMed] [Google Scholar]
- 39.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115: 610–621, 2005. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414: 454–457, 2001. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 41.Paragas N, Kulkarni R, Werth M, Schmidt-Ott KM, Forster C, Deng R, Zhang Q, Singer E, Klose AD, Shen TH, Francis KP, Ray S, Vijayakumar S, Seward S, Bovino ME, Xu K, Takabe Y, Amaral FE, Mohan S, Wax R, Corbin K, Sanna-Cherchi S, Mori K, Johnson L, Nickolas T, D’Agati V, Lin CS, Qiu A, Al-Awqati Q, Ratner AJ, Barasch J. α-Intercalated cells defend the urinary system from bacterial infection. J Clin Invest 124: 2963–2976, 2014. doi: 10.1172/JCI71630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paragas N, Qiu A, Hollmen M, Nickolas TL, Devarajan P, Barasch J. NGAL-siderocalin in kidney disease. Biochim Biophys Acta 1823: 1451–1458, 2012. doi: 10.1016/j.bbamcr.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin CS, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17: 216–222, 2011. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parikh CR, Dahl NK, Chapman AB, Bost JE, Edelstein CL, Comer DM, Zeltner R, Tian X, Grantham JJ, Somlo S. Evaluation of urine biomarkers of kidney injury in polycystic kidney disease. Kidney Int 81: 784–790, 2012. doi: 10.1038/ki.2011.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP. The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun 251: 625–631, 1998. doi: 10.1006/bbrc.1998.9514. [DOI] [PubMed] [Google Scholar]
- 46.Parravicini E, Lorenz JM, Nemerofsky SL, O’Rourke M, Barasch J, Bateman D. Reference range of urinary neutrophil gelatinase-associated lipocalin in very low-birth-weight infants: preliminary data. Am J Perinatol 26: 437–440, 2009. doi: 10.1055/s-0029-1214242. [DOI] [PubMed] [Google Scholar]
- 47.Petzold K, Poster D, Krauer F, Spanaus K, Andreisek G, Nguyen-Kim TD, Pavik I, Ho TA, Serra AL, Rotar L. Urinary biomarkers at early ADPKD disease stage. PLoS One 10: e0123555, 2015. doi: 10.1371/journal.pone.0123555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Preuss H, Geoly K, Johnson M, Chester A, Kliger A, Schreiner G. Tubular function in adult polycystic kidney disease. Nephron 24: 198–204, 1979. doi: 10.1159/000181715. [DOI] [PubMed] [Google Scholar]
- 49.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179–183, 1997. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 50.Raphael KL, Strait KA, Stricklett PK, Miller RL, Nelson RD, Piontek KB, Germino GG, Kohan DE. Inactivation of Pkd1 in principal cells causes a more severe cystic kidney disease than in intercalated cells. Kidney Int 75: 626–633, 2009. doi: 10.1038/ki.2008.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizk D, Chapman AB. Cystic and inherited kidney diseases. Am J Kidney Dis 42: 1305–1317, 2003. doi: 10.1053/j.ajkd.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Sabolić I, Brown D, Gluck SL, Alper SL. Regulation of AE1 anion exchanger and H+-ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int 51: 125–137, 1997. doi: 10.1038/ki.1997.16. [DOI] [PubMed] [Google Scholar]
- 53.Sallée M, Rafat C, Zahar JR, Paulmier B, Grünfeld JP, Knebelmann B, Fakhouri F. Cyst infections in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 4: 1183–1189, 2009. doi: 10.2215/CJN.01870309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 42: 658–676, 2003. doi: 10.1016/S0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 55.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002. doi: 10.1097/01.ASN.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- 56.Stiasny B, Ziebell D, Graf S, Hauser IA, Schulze BD. Clinical aspects of renal transplantation in polycystic kidney disease. Clin Nephrol 58: 16–24, 2002. doi: 10.5414/CNP58016. [DOI] [PubMed] [Google Scholar]
- 57.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators . Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres VE, Erickson SB, Smith LH, Wilson DM, Hattery RR, Segura JW. The association of nephrolithiasis and autosomal dominant polycystic kidney disease. Am J Kidney Dis 11: 318–325, 1988. doi: 10.1016/S0272-6386(88)80137-9. [DOI] [PubMed] [Google Scholar]
- 59.Torres VE, Keith DS, Offord KP, Kon SP, Wilson DM. Renal ammonia in autosomal dominant polycystic kidney disease. Kidney Int 45: 1745–1753, 1994. doi: 10.1038/ki.1994.227. [DOI] [PubMed] [Google Scholar]
- 60.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest 101: 1633–1642, 1998. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verlander JW, Madsen KM, Tisher CC. Structural and functional features of proton and bicarbonate transport in the rat collecting duct. Semin Nephrol 11: 465–477, 1991. [PubMed] [Google Scholar]
- 62.Virzi GM, Gastaldon F, Corradi V, de Cal M, Cruz DN, Clementi M, Ronco C. [Genotype-phenotype correlation in ADPKD: a possible role for NGAL?]. G Ital Nefrol 30: gin/30.4.7, 2013. [PubMed] [Google Scholar]
- 63.Virzi GM, Gastaldon F, Corradi V, de Cal M, Cruz DN, Clementi M, Ronco C. [Plasma NGAL and ADPKD progression]. G Ital Nefrol 32: gin/32.3.9, 2015. [PubMed] [Google Scholar]
- 64.Wall SM. Recent advances in our understanding of intercalated cells. Curr Opin Nephrol Hypertens 14: 480–484, 2005. doi: 10.1097/01.mnh.0000168390.04520.06. [DOI] [PubMed] [Google Scholar]
- 65.Wang E, Chiou YY, Jeng WY, Lin HK, Lin HH, Chin HJ, Leo Wang CK, Yu SS, Tsai SC, Chiang CY, Cheng PH, Lin HJ, Jiang ST, Chiu ST, Hsieh-Li HM. Overexpression of exogenous kidney-specific Ngal attenuates progressive cyst development and prolongs lifespan in a murine model of polycystic kidney disease. Kidney Int 91: 412–422, 2017. doi: 10.1016/j.kint.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Wilson PD. Polycystin: new aspects of structure, function, and regulation. J Am Soc Nephrol 12: 834–845, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Wu H, Chen L, Zhou Q, Zhang X, Berger S, Bi J, Lewis DE, Xia Y, Zhang W. Aqp2-expressing cells give rise to renal intercalated cells. J Am Soc Nephrol 24: 243–252, 2013. doi: 10.1681/ASN.2012080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Y, Xu JX, El-Jouni W, Lu T, Li S, Wang Q, Tran M, Yu W, Wu M, Barrera IE, Bonventre JV, Zhou J, Denker BM, Kong T. Gα12 is required for renal cystogenesis induced by Pkd1 inactivation. J Cell Sci 129: 3675–3684, 2016. doi: 10.1242/jcs.190496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 70.Yu AS, Kanzawa SA, Usorov A, Lantinga-van Leeuwen IS, Peters DJ. Tight junction composition is altered in the epithelium of polycystic kidneys. J Pathol 216: 120–128, 2008. doi: 10.1002/path.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 415: 389–395, 2002. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]