Abstract
Maintenance of normal epithelial ion and water transport in the lungs includes providing a thin layer of surface liquid that coats the conducting airways. This airway surface liquid is critical for normal lung function in a number of ways but, perhaps most importantly, is required for normal mucociliary clearance and bacterial removal. Preservation of the appropriate level of hydration, pH, and viscosity for the airway surface liquid requires the proper regulation and function of a battery of different types of ion channels and transporters. Here we discuss how alterations in ion channel/transporter function often lead to lung pathologies.
Keywords: chloride channels, oxidants, potasium channels, sodium channels
a thin fluid layer that forms a continuous barrier between the organism and external environment covers the lining of the lung. Although airway epithelia consist of various cell types with different morphologies and functions, one critical function is to control the volume, pH, and viscosity of this fluid layer to maintain normal lung function. This process requires that the airway epithelial cells must somehow govern the coordinated transport of solutes, ions, and water at their basal and apical surfaces. This organized transport is achieved by equipping epithelial cells with a variety of membrane solute, ion, and water transporters, differentially distributed along the apico-basal axis, among which sodium, chloride, and potassium channels/transporters play a critical role in maintaining lung homeostasis (Fig. 1). The functional significance of these channels is highlighted by realization that impairment of epithelial ion transport processes that alter the fluid composition/content are associated with a number of human diseases/pathologies, including cystic fibrosis (CF) (129), chronic bronchitis (22), chronic obstructive pulmonary disease (COPD) (249), and pulmonary edema (160). In this review, we focus on recent developments in understanding how ion channels maintain normal lung homeostasis.
Fig. 1.
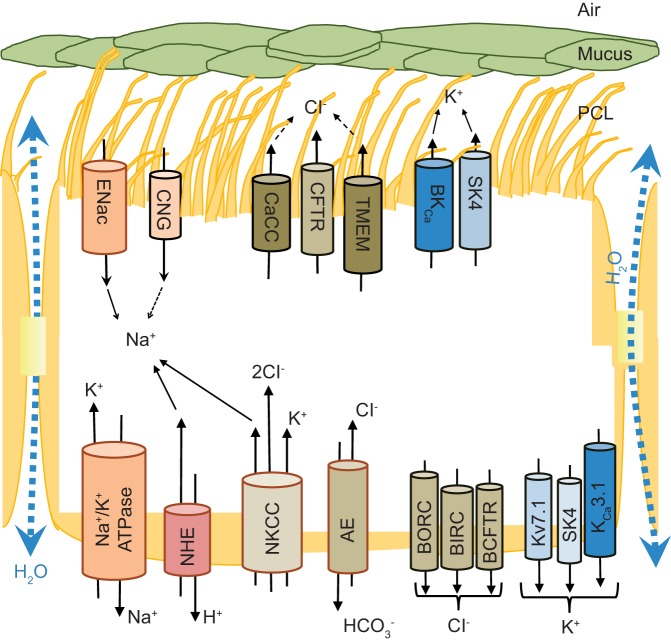
Ion channels and transporters that participate in regulation of lung periciliary liquid (PCL) homeostasis and mucociliary clearance by ciliated airway epithelia. Apical membrane sodium reabsorption, accompanied by chloride secretion along with passive H2O transport, provides the main mechanism responsible for PCL composition. The sodium reabsorption is mediated by coordinated function of epithelial sodium channel (ENaC, apical) and Na+-K+-ATPase (basal). However, apical cyclic nucleotide-gated channels (CNG) may contribute to sodium uptake as well. Furthermore, basal membrane located. CaCC, Ca2+-activated chloride channels; CFTR, cystic fibrosis transmembrane conductance regulator; TMEM, transmembrane domain; BKCa, large-conductance Ca2+-activated, voltage-dependent potassium channel; SK4, small-conductance Ca2+-activated potassium channels; NHE, Na+/H+ exchanger; NKCC, Na+-K+-Cl− cotransporter; AE, anion exchanger; BORC, basolateral outward rectifying channel; BIRC, basolateral inward rectifying channel; BCFTR, basolateral CFTR-like channel; Kv7.1, α-subunit of a voltage-dependent potassium channel; KCa3.1, Ca2+-activated potassium channel.
The majority of upper airway epithelial cells are cuboidal cells with cilia (219). These ciliated epithelial cells coordinate the beating of their cilia and govern ion transfer, and the efficiencies and fidelity of these two processes are necessary for proper mucociliary clearance (MCC). Efficient MCC requires that the airway surface liquid (ASL), which is composed of the mucous layer above and the periciliary liquid (PCL) below, is appropriately hydrated and of the right viscosity (mucus layer) to allow for the cilia to beat properly and move the mucus layer at an appropriate rate (142). MCC is a part of the innate immune system that is responsible for trapping and clearing the airways from inhaled pathogens and other noxious particles (142). Although the trapped pathogens and particles are transported within the mucus layer, the composition and thickness, pH, and viscosity of the PCL determines the optimal ciliary beat and thus the effectiveness of mucociliary clearance (156, 158, 217). The PCL characteristics are dependent on the active coordination between the cystic fibrosis transmembrane conductance regulator (CFTR), Ca2+-activated chloride channels (CaCC), chloride channel (CLC) 2, and the epithelial sodium channel (ENaC) (246). The fluid follows passively and results in the osmotic gradient that moves fluid from the airspace to interstitium [alveolar fluid clearance (AFC)] (156), and this regulates the ASL height (43, 217).
The apical cyclic nucleotide-gated channels (CNG) may support ENaC function, whereas CLC2 chloride channel and CaCC, including the transmembrane domain 16a (TMEM16) channels, contribute to apical chloride secretion (79, 191). Chloride secretion is sustained by the basolateral Na+-K+-2Cl− cotransporter and /Cl− anion exchanger, whereas basolateral Na+/H+ exchanger (NHE) maintains intracellular pH (74). Additionally, the basolateral membrane contains chloride channels [basolateral outward rectifying channel (BORC), basolateral inward rectifying channel (BIRC), and basolateral CFTR-like channel (BCFTR)] that along with basolateral- and apical membrane-located potassium channels {basolateral α-subunit of a voltage-dependent potassium channel (Kv7.1) to K7.5 and Ca2+-dependent potassium channels [small-conductance Ca2+-activated potassium channels (SK4), large-conductance Ca2+-activated, voltage-dependent potassium channel (BKCa), and Ca2+-activated potassium channel (KCa3.1)]} may modulate apical chloride secretion (9, 42, 68, 103, 139).
Finally, the epithelial cells that form the alveolar epithelium (AE) are crucial for efficient gas exchange and are separated from the gas phase by a thin layer of alveolar lining fluid (ALF). The amount, volume, and composition of ALF are crucial for gas diffusion, and therefore the electrolyte composition of ALF is strictly controlled by AE-mediated ion transport. For example, amiloride inhibition of sodium reabsorption in AE results in a significant reduction of basal fluid clearance in human lungs (159, 160). The AE consist of two types of epithelial cells, alveolar type I (ATI) and alveolar type II (ATII) cells, that mediate vectorial ion processes (49, 58, 239) despite their differences in function and morphology (49, 70). Both ATI and ATII express ENaC, amiloride-insensitive CNG cation channels (239), potassium channels, CFTR, γ-aminobutyric acid type A (GABAA) channels, and voltage-gated chloride channels (CLC2 and CLC5) (103). Furthermore, ATI cells express aquaporin 5 (AQP5) and exhibit the highest water permeability of any mammalian cell type (58). As in ciliated epithelial cells, fluid removal out of alveoli in the interstitium uses a transepithelial osmotic gradient created in AE cells by sodium uptake through the coordinated action of apical ENaC (117, 155, 172), CNG cation channels (239), and the basal ouabain-sensitive Na+-K+-ATPase (197). However, the mechanism for chloride and potassium transport by AE cells is less clear (170). AT II cells are also important for enriching the ALF with surfactant (83, 105, 199, 225) and immunoreactive proteins (27, 48, 128, 211) which facilitate innate immune responses.
Role of Membrane Potential
The membrane potential of the apical membrane (Va) is governed by its major conductances, which are the apical sodium and chloride conductances, whereas the basolateral membrane potential (Vb) is largely governed by its potassium conductance. The primary ion transport function of lung epithelia is sodium absorption across apical sodium channels driven by the basolaterally located Na+-K+-ATPase. Recycling of potassium across the basolateral membrane results in a hyperpolarization of the cell, and chloride distributes passively across the apical membrane according to its electrochemical driving forces. Although there are a number of other ion channels expressed in lung epithelium, including the apical hydrogen ion channel, their contributions to the membrane potentials can be safely neglected by the high sodium and chloride conductances (67, 155).
The differential expression of the major ion conductances in the two membranes and the selective ion gradients toward the mucosal and the serosal compartment result in distinct electromotive forces across the two membranes. However, the difference in the membrane potentials of the apical and basolateral membrane is solely caused by the junctional resistance, Rj, where the voltage difference between Va and Vb drops. Thus, the tightness of the epithelial monolayer determines the depolarization of Va with respect to Vb. In leaky epithelia, where Rj is small compared with Ra + Rb, there is little difference between Va and Vb, and as a result the transepithelial potential, Vt, is small (67).
In alveolar cells, in comparison, membrane potentials have been little studied because of their complicated anatomy in vivo and the lack of good cell culture models for alveolar epithelia. Stimulation of type II monolayers with a cAMP agonist activates both apical CFTR chloride channels and ENaC sodium channels and results in a transepithelial hyperpolarization. Indeed, Lazrak and Matalon in their study in human lung ATI-like cells (H441) observed that stimulation with cAMP leads to sodium entry and thus the membrane depolarization (137). Hence, it can be concluded that maintaining proper membrane potential requires the coordinated modulation of potassium and choride ion channel activities.
Sodium Transport
The driving force for apical sodium reabsorption in airway epithelial cells is provided by the Na+-K+-ATPase located along the basolateral membrane (50). The Na+-K+-ATPase is composed of a heterodimer of two subunits (α and β) and small accessory transmembrane proteins of the FXYD family (66). The conversion of one ATP to ADP results in transport of three sodium ions out of the cell in exchange for two potassium ions pumped into the cell (213). The Na+-K+-ATPase activity is stimulated by sodium and potassium ions from the cytoplasmic and extracellular side, respectively (66). However, because potassium and sodium compete at the cytoplasmic site, an acute increase in intracellular sodium stimulates Na+-K+-ATPase activity and leads to a rapid export of intracellular sodium (66). Furthermore, the sodium activation of the Na+-K+-ATPase is strongly cooperative, and small changes in intracellular sodium levels lead to changes in Na+-K+-ATPase activity. Thus, under physiological conditions, intracellular sodium is rate limiting for Na+-K+-ATPase activity, whereas the intracellular ATP concentration is not. ATP may become rate limiting, however, when its synthesis is decreased under pathological conditions such as during periods of hypoxia (121, 132, 181) or oxidative stress (230). Furthermore, during acute lung injury, the accumulation of a protein-rich edema impairs gas exchange and leads to hypoxemia that is accompanied by a decrease of Na+-K+-ATPase function (234). Hypoxia reduces alveolar fluid clearance and the nasal potential difference (35, 252). In alveolar epithelial cells exposed to severe hypoxia, an increased production of mitochondrial reactive oxygen species (ROS) leads to Na+-K+-ATPase endocytosis and ubiquitin-dependent degradation (138). Additionally, the basal membrane of ciliated cells contains ATP-dependent amiloride-sensitive electroneutral NHE channels (176, 177) that are involved in maintaining intracellular pH balance (74).
Alveolar edema clearance requires active sodium transport that is mediated by Na+-K+-ATPases (161, 224). Under normal conditions, this pump is therefore required for maintenance of a dry alveolar space. Furthermore, downregulation of alveolar Na+-K+-ATPase promotes pulmonary edema in models of lung injury, and therefore activation of dopaminergic or adrenergic receptors that increase Na+-K+-ATPase activity provides a therapeutic potential for increasing fluid clearance from the alveolar space (161, 166, 224). The obvious importance of this pump is clearly reflected in its ability to resolve alveolar edema under pathological conditions (159).
The main mediator of transcellular sodium reabsorbtion is ENaC that is found at the apical membrane of human airway epithelia. Although it is well established that ENaC forms a trimer consisting of α-, β- and γ-subunits (33), a novel δ-subunit has been identified in both epithelial and nonepithelial tissues (including human lung) (86, 110). Because the δ-subunit is able to form an amiloride-sensitive functional channel along with β- and γ-subunits (236), at least two different channel compositions are possible (110). The δ-subunit-containing ENaC channels have been shown to be responsible for up to one-half of the amiloride-sensitive transport in human nasal epithelial cells (8). The ENaC complexes consisting of other combinations besides the usual α-β-γ complex, however, exhibit lower selectivity of sodium over potassium and are less sensitive to amiloride (38). Although deregulation of the δ-subunit is associated with a predisposition to respiratory diseases (110), the determination of the exact role of this subunit in maintaining ion lung homeostasis is limited because of the absence of δ-subunit expression in mice (87). Because ENaC is often considered the rate-limiting step of lung sodium homeostasis, it is not surprising that changes in sodium channel function that result from epigenetic or pathological factors (reviewed in Ref. 156) often contribute to lung disease. To date, numerous physiological (7, 41, 62, 73, 76, 78, 82, 89, 90, 108, 122, 192, 226), environmental (54, 60, 98, 111, 162, 231), and genetic [mutations in ENaC subunits (188)] factors have been shown to modulate ENaC activity in lung epithelium and thus affect ASL balance (see Table 1) (156).
Table 1.
Factors that elevate or inhibit ENaC channel expression and/or function in alveolar cells and thus affect ASL homeostasis
| Factor | Impact on ENaC Function and ASL | Ref. No. |
|---|---|---|
| SPLUNC1 | SPLUNC1 is highly expressed in the airways and secreted on mucosal surfaces as a volume sensor that regulates ENaC activity and mucosal volumes, including that of the airway surface liquid. SPLUNC1 binds to ENaC, preventing ENaC cleavage and activation by serine proteases. | 82, 101, 125, 126, 242 |
| Female sex hormones | Estradiol and progesterone directly regulate expression and activity of alveolar ENaC and ASL volume. Estradiol increases ENaC channel activity and apical plasma membrane abundance of the αENaC through G protein-coupled estrogen receptors. | 90, 108 |
| β-Agonists and neurotransmitters | β-Adrenergic stimulation enhances fluid absorption mediated by ENaC stimulation. In the submucosal glands, however, increased cAMP from β-adrenergic receptor stimulation increases ion and fluid secretion and facilitates mucociliary clearance. β2-Agonists and dopamine activate ENaC channels in ATII cells. The muscarinic agonists, carbachol and oxotremorine, activate epithelial ENaC via a RhoA/ROCK signaling pathway in ATII cells. | 7, 89, 226 |
| Cytokines | IL-4 may favor the hydration of the airway surface by decreasing Na+ absorption and increasing Cl− secretion to hydrate the mucus, which is hypersecreted during inflammatory conditions. | 53, 54, 73, 76–78, 122, 146, 180, 192 |
| IL-1β increases lung epithelial and endothelial permeability. IL-1β reduces αENaC expression and activity through the activation of p38 MAPK in ATII cells. p38 MAPK-dependent inhibition of αENaC promoter and an alteration in ENaC trafficking to the apical membrane of ATII cells reduces ion and water transport across the lung. | ||
| TGF-β1 has an inhibitory effect on Na+ uptake and αENaC expression in ATII cells that is mediated by ERK1/2-dependent inhibition of the αENaC promoter activity. The recent studies proposed a mechanism in which TGF-β rapidly and sequentially activates phospholipase D1, phosphatidylinositol-4-phosphate 5-kinase 1α, and NOX4 to produce ROS, driving internalization of βENaC, the subunit responsible for cell surface stability of the αβγENaC complex. TNF-α, a cytokine present during lung infection, has a profound influence on the capacity of alveolar epithelial cells to transport Na+. TNF-α decreased the expression of the α-, β-, and γ-subunits of epithelial ENaC mRNA and reduced αENaC protein and ENaC activity. Furthermore, the lectin-like domain of TNF (mimicked by the TIP peptide) was shown to improve ALC in models of both hydrostatic and permeability edema. The TIP peptide directly binds to the α-subunit of ENaC and stabilizes the channel’s complex formation with myristoylated alanine-rich C kinase substrate and phosphatidylinositol 4,5-bisphosphate, which are essential for the open conformation, in the presence of pneumococcal pneumolysin, an important mediator of permeability edema. | ||
| PKC | Activation of PKC has been shown to reduce ENaC activity, and, conversely, inhibition of PKC has been observed to increase ENaC function in ATII cells. | 47, 48 |
| uPA | Urokinase upregulates ENaC activity and fluid reabsorption in vivo and in vitro. The proposed mechanisms for the regulation of ENaC by uPA include augmentation of Na+-K+-ATPase, proteolysis, and inhibition of ERK1/2 phosphorylation. | 41 |
| ROS | GSH serves as a main antioxidant in the lungs, and changes in GSH redox potential have an effect on ENaC activity. ROS GSSG impairs ENaC function and alveolar fluid clearance in vivo. Hence, ROS through increases in GSSG reduce ALF. | 60 |
| Hypoxia | Although the results of several studies suggest that severe alveolar hypoxia results in decreased AFC and Na+ transport, the molecular mechanisms underlying this observation require further studies. Furthermore, chronic hypoxia leads to increased ROS formation that may contribute to observed impaired ENaC activity as well. | 98, 173, 231 |
| Hypercapnia | Hyperercapnia (elevated CO2 levels) has been shown to impair AFC, thereby causing retention of pulmonary edema and may lead to worse outcomes; hypercapnia affects ENaC cell surface stability by stimulation of polyubiquitination of βENaC and subsequent endocytosis of the α/βENaC complex in AECs. Hypercapnia induced ubiquitination and cell surface retrieval of ENaC and was critically dependent on phosphorylation of the Thr615 residue of βENaC, which was mediated by ERK1/2. The hypercapnia-induced ENaC dysfunction may contribute to impaired alveolar edema clearance. | 92, 94, 95 |
| LPS | LPS, a glycolipid abundant in the outermost membrane of Gram-negative bacteria, is a potent proinflammatory molecule. LPS has been reported to decrease ENaC activity and expression of αENAC in AEC. It has been postulated that LPS attenuates αENaC mRNA expression via decreased transcription. | 162 |
SPLUNC1, short-palate lung and nasal epithelial clone 1; ENaC, epithelial sodium channel; ASL, airway surface liquid; ROCK, Rho kinase; ATII, alveolar type II; IL, interleukin; MAPK, mitogen-activated protein kinase; TGF-β1, tumor growth factor-β1; NOX4, NADPH oxidase 4; TNF-α, tumor necrosis factor-α; TIP, TIP peptide of the lectin-like domain of TNF-α; PKC, protein kinase C; uPA, urokinase-like plasminogen activator; ROS, reactive oxygen species; GSH, glutathione; GSSG, oxidized glutathione; ALF, alveolar lining fluid; AFC, alveolar fluid clearance; AEC, alveolar epithelial cells; ERK, extracellular signal-regulated kinase; LPS, liposaccharide. Na+/H+ exchanger is responsible for preserving intracellular pH. The apical chloride secretion is mediated mainly by cystic fibrosis transmembrane conductance regulator (CFTR); however, Ca2+-dependent apical channels like transmembrane domain 16a contribute as well. Chloride secretion is maintained by the basolateral Na+-K+-2Cl− cotransporter and /Cl− anion exchanger. Furthermore, other basolateral chloride channels, including basolateral outward rectifying channel, basolateral inward rectifying channel, and basolateral CFTR-like channel, have been proposed to modulate apical chloride secretion. Basolateral voltage-dependent potassium channels (Kv7.1–Kv7.5) as well as basolateral and apical Ca2+-dependent potassium channels (small-conductance Ca2+-activated potassium channels; large-conductance Ca2+-activated, voltage-dependent potassium channels; Ca2+-activated potassium channels) have also been proposed to modulate apical chloride secretion.
Many of these ENaC-modulating factors, especially those using general signaling pathways such as protein kinase A, mitogen-activated protein kinase, extracellular signal-regulated kinase (ERK), or PKC, are ubiquitous and regulate a wide range of cellular activities (157, 243). Until recently, it has proven to be particularly difficult to use ENaC modulation as a potential therapy for lung diseases such as CF. Nevertheless, a deeper understanding of the molecular and physiological mechanisms that modulate airway sodium homeostasis remains a viable target for potential therapies. For example, the short palate lung and nasal epithelial clone 1 (SPLUNC1), a peptide inhibitor of ENaC that is endogenously secreted in ASL, functions as ENaC activity modulator in response to changes in ASL volume (101). Following any increase in ASL volume, this soluble ENaC inhibitor concentration decreases, and this allows for ENaC activation and increased sodium resorption, whereas, with a reduction in ASL volume, the SPLUNC1 peptide is concentrated and inhibits ENaC activation (101, 126).
The recent identification of the SPLUNC1 ENaC-inhibitory domain has contributed to the design of novel specific ENaC peptide inhibitors that have been shown to prevent sodium hyperabsorption in CF lungs (101, 136, 141). The therapeutic potential of ENaC inhibition is further supported by a recent study showing that use of a specific ENaC blocker could lead to ASL rehydration and increased ciliary function and mucociliary clearance (5). These data imply that ENaC inhibition may also be an effective strategy to treat other lung diseases such as COPD. Finally, because ENaC requires proteolytic cleavage of its extracellular loops by specific extracellular proteases for activity (127), it is possible that, in conditions of chronic neutrophilia present in CF and COPD lungs, the increased levels of human neutrophil elastase (HNE) may nonspecifically activate ENaC and contribute to enhanced sodium absorption and ASL dehydration (32). Nonantibiotic macrolides that prevent HNE activity could be used for limiting ENaC activity and thus may be useful in CF and COPD therapies (227). Inter-α-inhibitor (IαI), a serum protease inhibitor consisting of three polypeptides and known to be present in the alveolar spaces after lung injury and in CF, also inhibits ENaC by preventing its cleavage by serine proteases but does not affect CFTR function. A single intranasal instillation of IαI in ∆F508 mice decreased ENaC-nasal potential difference 24 h later by 25% (136).
AE cells contain numerous antioxidants, such as glutathione and ascorbate, that protect cells from oxidants (34, 59). Oxidative stress is a concern because increased levels of ROS that can affect lung redox potential are observed in numerous lung pathologies, including COPD, asthma, lung cancer, and acute lung injury (ALI), and are often seen during influenza infections (39, 69, 133, 178, 185, 229). Recent studies have also shown that exposure to high levels of ROS results in increased amounts of oxidized glutathione in the ALF, and this reduces the ENaC activity in primary AE cells and impairs the lung fluid balance (60). This last observation suggests that antioxidant-based therapies should be considered.
Besides ENaC, the apical membrane-located CNG cation channels may provide another sodium entry pathway in lung epithelial cells (93, 239). These nonselective amiloride-insensitive CNG channels were detected in both ATI and ATII cells, show no preference for sodium over potassium, and can be activated by micromolar concentrations of cGMP (123, 239). Because exogenous cGMP was shown to stimulate liquid absorption, the CNG channels have been proposed to play a role in the regulation of ALF volume (93, 239). However, the exact role of these channels in the regulation of lung fluid homeostasis remains poorly understood (93, 119, 120).
Chloride Transport
The apically located cAMP-dependent CFTR chloride (3) and bicarbonate channel (183) is responsible for a large part of the airway chloride secretion in humans (47). The importance of this channel for lung homeostasis is clearly illustrated by its role in CF, where loss of CFTR function leads to dehydrated mucus, airway obstruction, chronic bacterial infections, inflammatory responses, and eventually bronchiectasis and respiratory failure (174, 194). Furthermore, the decreases in CFTR function have been proposed to play an important role in the pathology of COPD as well (61, 184, 187, 193).
Given that the CFTR-driven apical chloride secretion and ENaC-mediated sodium reabsorption are critical for maintaining PCL homeostasis, it is not surprising that these two activities need to be balanced relative to each other and that one activity may regulate the activity of the other. This hypothesis is supported by the observation that airway epithelia of CF patients have elevated amiloride-sensitive sodium reabsorption, illustrating that CFTR regulates ENaC activity (23, 107, 129, 131). Furthermore, studies in CFTR knockout mice demonstrate the same elevated ENaC activity (135). Interestingly, in contrast to this hypothesis, in the CF pig model, loss of CFTR does not result in increased transepithelial sodium absorption (37). Additionally, the question of whether ENaC regulates CFTR activity remains unclear (46). Furthermore, understanding the mechanism of the ENaC-CFTR cross talk in airway epithelia is complicated by the fact that CFTR activity and ENaC function are often affected by the same physiological [β-agonists (7, 179)] and environmental factors that include oxidative stress (19, 20, 175, 203, 218, 248), hypoxia (36, 244), inflammatory responses (28, 30, 169, 202, 241), and influenza infections (102, 109, 144, 189, 235).
The apical side of airway epithelia contains other channels besides CFTR that contribute to chloride secretion. These include the chloride voltage-gated channel 2 (CLCN2) and Ca2+-activated ion channels (CaCCs), including TMEM16 family members that have been shown to contribute to apical chloride secretion (79, 191, 198). Although the extent of TMEM16A channel contribution to Ca2+-induced chloride secretion in airways is limited (167, 200), the mechanism of Ca2+-activated chloride secretion requires further analysis. Recent studies, however, have shown that the inhibition of these channels along with the modulation of Na+-K+-Cl− cotransporter (NKCC) activity leads to smooth muscle relaxation and thus, along with β-agonists, provides a novel basis for therapy in asthma (55, 56). Furthermore, gaining control over CLCN2 or CaCC activity may provide an alternative approach to CFTR loss and thus provide a potential therapeutic benefit for CF patients (99, 198). On the other hand, activation of TMEM16A following exposure of airway smooth muscle cells to oxidant gases results in membrane depolarization that may contribute to the activation of RhoA and airway smooth muscle contraction (134).
During development, ClCN2 is highly expressed in lung tissue and has been proposed as a potential pathway to replace the missing chloride conductance in patients suffering from CF (204), However, Clcn2−/− mice do not suffer from lung disease, and additional ClCN-2 knockout in CFTR−/− mice did not deteriorate the phenotype of CF (245).
TMEM16A epithelial expression is increased in asthmatic patients, particularly in secretory cells, and inhibition of TMEM16A-CaCC activity significantly impairs mucus secretion in primary human airway surface epithelial cells. Furthermore, inhibition of TMEM16A-CaCC significantly reduces mouse and human airway smooth muscle (ASM) contraction in response to cholinergic agonists (104). Furthermore, increased expression of TMEM16A was observed in the airways of CF patients, suggesting that TMEM16A expression/function is upregulated in CF lung disease, possibly as a response to the presence of bacteria in the airways (31).
NKCC mediates chloride entry across the basolateral membranes of secretory epithelia. The driving force for cellular chloride entry and accumulation is supplied primarily by the inward chemical gradient of sodium established by the pump; the basolateral membrane potential does not influence this electrically neutral process. Ultimately, the rate of basolateral chloride entry via NKCC determines the overall rate of chloride secretion. The timing of NKCC activation generally follows that of CFTR and the potassium channels, resulting in some loss of cellular chloride and potassium and a decrease in the cell volume (74). NKCC is upregulated in response to Gram-negative bacterial toxins like lipopolysaccharide (LPS) in the lung. Recently, basolateral NKCC1 and apical-expressed CFTR were shown to be critical for the reversal of fluid uptake in a secretory alveolar epithelium by driving chloride secretion. It was reported that an acute increase in left atrial pressure decreases amiloride-sensitive sodium uptake across the alveolar epithelium and concomitantly stimulates sodium and chloride uptake via basolateral NKCC and chloride secretion in the alveolar space via apical CFTR, thus effectively reversing sodium-driven AFC in chloride-driven alveolar fluid secretion (AFS). Importantly, inhibition of CFTR and NKCC1 improved AFC and attenuated edema formation (238). This was also the case in influenza infection: Wolk et al. reported that inhibition of CFTR decreased alveolar edema in influenza infection (240). Others, however, have reported that influenza viruses and its M2 protein decrease CFTR activity and levels in the apical membranes of epithelial cells (91, 143, 145).
Despite the continuous development of novel therapies for CF (17, 36, 45, 47, 51, 52, 75, 84, 163, 196, 215), the vast majority treats the symptoms rather than correcting molecular defects in the channel. Correcting the CFTR channel has been difficult because, besides the most common mutation, ∆F508 CFTR, >2,000 mutations in the CFTR gene have been identified (11, 195). Translating this complex genetic CF background into CFTR protein structure and function has not proven to be an easy task. In that regard, a recent high-resolution structure of the human CFTR protein (3.9-Å as determined by electron cryomicroscopy) was presented that may provide new insights into channel function (140). The structure revealed a previously unresolved helix belonging to the R domain that docked inside the intracellular vestibule that blocks channel opening (140). Furthermore, a structure of the purified G551D CFTR, a channel-gating mutant, has also been determined by electron cryomicroscopy (72), suggesting that this type of methodology is being used more and more. And, finally, another recent study identified extracellular loop 1 (ECL1) of CFTR as critical for proper channel function (106). These studies increase our understanding of structural basis of chloride channel function (71, 140, 247) in CFTR and will likely aid in understanding the effects of mutations in CFTR (44) and hopefully allow for the development of more effective therapies.
Less effort, however, has been placed on studying chloride movement across the basolateral membrane. Basolateral chloride transport appears to be mediated by the outwardly rectifying chloride channel (68), the inwardly rectifying chloride channel (68), and a cAMP-dependent low-conductance linear CFTR-like channel (42, 68). Furthermore, the action of these channels is facilitated by electroneutral channels such as the NKCC cotransporter (228) and the /Cl− exchanger (14) that provide a sufficient intracellular supply of chloride ions (68).
Although the CFTR function in ciliated epithelial cells is well established, the role of this channel in AE is less clear. Despite the fact that numerous studies identified CFTR activity in AE [both in ATI (116) and ATII (65) cells] and proposed this channel to be involved in ASL regulation (65, 116), there is no consensus as to whether CFTR is responsible for chloride absorption (64, 65, 112) or secretion (25, 214). Furthermore, a recent study indicated that CFTR was at the basolateral membrane of AE cells (15). These controversies may partially be explained from limited availability of these cells and various culturing conditions that were used to isolate and culture these cells (57). There are other channels in AE besides CFTR that include electroneutral cotransporters like NKCC (96, 212), K+-Cl− (KCC) (212) and the /Cl− exchanger (14), as well as the GABAA chloride channel (113, 114), and the voltage-gated CLC2 and CLC5 (115). All of these channels and transporters have been proposed to be involved in transepithelial chloride transport.
Potassium Transport
The wide variety of diverse potassium channels [over 30 (9)] that are apically and basolaterally located in airway epithelia maintain the electrochemical gradient and thus support lung ion and fluid homeostasis (9, 139). Changes in activity of airway epithelial basolateraly located potassium channels (including KCNQ voltage-activated Na+–Kv7 channels) were shown to impact apical cAMP-dependent chloride secretion (26, 149, 150) and Ca2+-dependent chloride secretion (16). To date, the presence of all three classes of Ca2+-dependent potassium channels [big-conductance potassium (BK), intermediate-conductance potassium (IK)/KCa3.1, and small-conductance potassium (SK)] was confirmed at the basolateral membrane of airway epithelial cells (130, 149, 232). Furthermore, some apically located Ca2+-dependent potassium channels, including SK4-like channels (16) and BKCa (152, 153), have a significant influence on chloride secretion and thus are important for MCC and ASL volume regulation.
Importantly, the role of the potassium channels in AE cells is not limited to sodium reabsorption maintenance. The activity of ATP-sensitive potassium channels in alveolar cells strongly relies on intracellular ATP levels and thus links the membrane potential with the metabolic state of the cells (168, 233). Additionally, a recent study reported that the two-pore-domain potassium channel Trek-1 regulates release of proinflammatory interleukin-6 (IL-6) from AE cells (18, 206–208). This suggests that AE cells may use potential potassium channels to adapt the membrane potential in response to stress factors and thus are crucial for airway epithelial repair (29, 233). Hence, understanding their physiological role in airway regeneration and in inflammatory responses may be important for lung pathologies such as CF, ALI, and acute respiratory distress syndrome (208, 209).
Additionally, potassium channels act in alveolar epithelium as oxygen sensors and thus adjust lung function to environmental changes in O2 levels (9, 124, 170, 171, 209). Alveolar potassium channels, in particular BKCa, that have been shown to reduce their opening time during hypoxia (118) are able to detect O2 variation and modulate their activity to adjust ion transport and fluid clearance (9, 124, 170, 171). Although the molecular mechanism(s) governing oxygen sensing by alveolar potassium channels remains unclear, their properties make them potential therapeutic targets for lung diseases associated with hypoxia that include pulmonary hypertension (147, 209).
Moreover, the involvement of potassium channels has been proposed in respiratory conditions such as asthma and chronic COPD (148). Pulmonary hypertension can be caused by a defect in the function of potassium channels or by alveolar hypoxia. Importantly, hypoxia selectively inhibits the function and expression of voltage-gated potassium (KV) channels in pulmonary arterial smooth muscle cells (SMCs). The activity of potassium channels regulates the membrane potential (Em) of SMCs, which in turn regulates the cytoplasmic free Ca2+ concentration ([Ca2+]cyt). Depolarization of the Em leads to an elevated [Ca2+]cyt by opening voltage-dependent Ca2+ channels. Elevated [Ca2+]cyt is implicated in stimulating vascular SMC proliferation and inducing vasomotor tone and, hence, vasoconstriction. Vasoconstriction causes elevation of intravascular pressure and elastic stretch of the SMCs, both of which have been shown to play a role in pulmonary arterial cellular growth and synthetic activity, creating a vicious cycle of cellular hypertrophy, proliferation, and vascular remodeling. Dysfunction of potassium channels has also been linked to decreased apoptosis in pulmonary arterial SMCs, a condition that contributes further to the medial hypertrophy of the arterial walls and vascular remodeling (21, 151, 165, 190). Furthermore, KCNRG, a putative potassium channel-regulating protein expressed in bronchial epithelial cells, was identified as an autoantigen in autoimmune polyendocrine syndrome type 1 (APS-1) associated with pulmonary manifestations (1).
Role of Ca2+ Transport
As discussed above, both the chloride and potassium ion transport in airway epithelial cells rely to some extent on Ca2+-dependent channel activity (e.g., CaCCs and BKCa). Some support for this idea comes from the fact that the proper folding and membrane expression of crucial transmembrane ion channels like CFTR (12, 13, 154) relies on the endoplasmic reticulum being the main cellular Ca2+ reservoir (4, 10, 201). Furthermore, a recent study showed that the extracellular Ca2+-sensing receptor regulates human fetal lung development via CFTR (24).
A variety of environmental insults such as hypoxia (18, 85, 88, 182, 237, 251), oxidative stress (134, 220, 221, 223), and inflammation (2, 40, 63, 100, 250) that affect cellular Ca2+ homeostasis were shown to affect Ca2+ channel activity in airway epithelium as well. Because changes in airway Ca2+ transport can be reflected in Ca2+-dependent potassium and chloride channel activity, Ca2+ channels provide an additional mechanism that governs lung ion and fluid homeostasis. They are therefore emerging as a potential novel drug target for lung pathologies, including asthma (80, 216) and chronic inflammation (2, 40, 250), oxidative lung injury (81, 134, 164, 223), and CF (100). Importantly, the transient receptor potential vanilloid-4 channel (TVRPV4), a nonselective Ca2+ channel that responds to variety of stimuli (164, 186, 221, 222), governs the production of a number of proinflammatory mediators in airway epithelia. Thus, anti-inflammatory inhibitors of TRPV4 have been proposed as a therapeutic target for lung injury, CF (100), and acute respiratory distress syndrome (6, 221). Because of the concomitant pulmonary or systemic infections that accompany patients with these lung pathologies, the use of TRPV4 inhibitors, unfortunately, carries the risk of compromising immune responses (164). Maintaining the proper intracellular Ca2+ levels and Ca2+ transport by airway myocytes that control the extent of airway opening (97, 210) is often underappreciated and critical for normal lung function.
Current Limitations and Challenges
The proper maintenance of airway epithelium ion balance is imperative for healthy lung homeostasis. However, the complete characterization of biochemical and biophysical properties and the coordinated function of ion channels that govern lung function still remain a challenge. The apical and basal membranes have different sets of ion transporters, and different cell types have different channels as well, which adds to the cellular/tissue complexity. Hence, even understanding channel function by itself and its relation to ion exchange makes picking specific molecular targets for therapeutic approaches difficult at best and problematic at worst. Although the recent reports have increased our knowledge of lung epithelial ion transport, they have also exposed how limited our understanding is on the relationships between ion channels, intracellular signaling networks, and environmental factors as well (205). That being said, recent studies on the role of airway epithelial channels have provided promising new drug targets.
Concluding Remarks
The mechanisms that control ion homeostasis in airways rely on the coordinated action of an entire network of channels, and this is reflected in the complex pathological features of the majority of lung diseases. Hence, understanding the rules that govern these channel interactions within this network of airway ion regulation remains an important challenge to overcome for achieving successful interventions in various lung airway diseases.
GRANTS
This research was supported by the CounterACT Program, National Institutes of Health Grants 5U01-ES-026458 02 (S. Matalon), 1 U01-ES-027697 01 (S. Matalon), and P30-DK-072482 (J. F. Collawn), and by the National Science Center OPUS Program under contract 2015/17/B/NZ3/01485 (R. Bartoszewski).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.B. drafted manuscript; R.B., S.M., and J.F.C. edited and revised manuscript; R.B., S.M., and J.F.C. approved final version of manuscript.
REFERENCES
- 1.Alimohammadi M, Dubois N, Sköldberg F, Hallgren A, Tardivel I, Hedstrand H, Haavik J, Husebye ES, Gustafsson J, Rorsman F, Meloni A, Janson C, Vialettes B, Kajosaari M, Egner W, Sargur R, Pontén F, Amoura Z, Grimfeld A, De Luca F, Betterle C, Perheentupa J, Kämpe O, Carel JC. Pulmonary autoimmunity as a feature of autoimmune polyendocrine syndrome type 1 and identification of KCNRG as a bronchial autoantigen. Proc Natl Acad Sci USA 106: 4396–4401, 2009. doi: 10.1073/pnas.0809986106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaparti V, Pascoe CD, Jha A, Mahood TH, Ilarraza R, Unruh H, Moqbel R, Halayko AJ. Tumor necrosis factor regulates NMDA receptor-mediated airway smooth muscle contractile function and airway responsiveness. Am J Physiol Lung Cell Mol Physiol 311: L467–L480, 2016. doi: 10.1152/ajplung.00382.2015. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253: 202–205, 1991. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- 4.Antigny F, Norez C, Becq F, Vandebrouck C. Calcium homeostasis is abnormal in cystic fibrosis airway epithelial cells but is normalized after rescue of F508del-CFTR. Cell Calcium 43: 175–183, 2008. doi: 10.1016/j.ceca.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Åstrand AB, Hemmerling M, Root J, Wingren C, Pesic J, Johansson E, Garland AL, Ghosh A, Tarran R. Linking increased airway hydration, ciliary beating, and mucociliary clearance through ENaC inhibition. Am J Physiol Lung Cell Mol Physiol 308: L22–L32, 2015. doi: 10.1152/ajplung.00163.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banga A, Flaig S, Lewis S, Winfree S, Blazer-Yost BL. Epinephrine stimulation of anion secretion in the Calu-3 serous cell model. Am J Physiol Lung Cell Mol Physiol 306: L937–L946, 2014. doi: 10.1152/ajplung.00190.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangel-Ruland N, Sobczak K, Christmann T, Kentrup D, Langhorst H, Kusche-Vihrog K, Weber WM. Characterization of the epithelial sodium channel delta-subunit in human nasal epithelium. Am J Respir Cell Mol Biol 42: 498–505, 2010. doi: 10.1165/rcmb.2009-0053OC. [DOI] [PubMed] [Google Scholar]
- 9.Bardou O, Trinh NT, Brochiero E. Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L145–L155, 2009. doi: 10.1152/ajplung.90525.2008. [DOI] [PubMed] [Google Scholar]
- 10.Bartoszewska S, Kochan K, Madanecki P, Piotrowski A, Ochocka R, Collawn JF, Bartoszewski R. Regulation of the unfolded protein response by microRNAs. Cell Mol Biol Lett 18: 555–578, 2013. doi: 10.2478/s11658-013-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartoszewski R, Króliczewski J, Piotrowski A, Jasiecka AJ, Bartoszewska S, Vecchio-Pagan B, Fu L, Sobolewska A, Matalon S, Cutting GR, Rowe SM, Collawn JF. Codon bias and the folding dynamics of the cystic fibrosis transmembrane conductance regulator. Cell Mol Biol Lett 21: 23, 2016. doi: 10.1186/s11658-016-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartoszewski R, Rab A, Fu L, Bartoszewska S, Collawn J, Bebok Z. CFTR expression regulation by the unfolded protein response. Methods Enzymol 491: 3–24, 2011. doi: 10.1016/B978-0-12-385928-0.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartoszewski R, Rab A, Twitty G, Stevenson L, Fortenberry J, Piotrowski A, Dumanski JP, Bebok Z. The mechanism of cystic fibrosis transmembrane conductance regulator transcriptional repression during the unfolded protein response. J Biol Chem 283: 12154–12165, 2008. doi: 10.1074/jbc.M707610200. [DOI] [PubMed] [Google Scholar]
- 14.Begenisich T, Melvin JE. Regulation of chloride channels in secretory epithelia. J Membr Biol 163: 77–85, 1998. doi: 10.1007/s002329900372. [DOI] [PubMed] [Google Scholar]
- 15.Berger J, Richter K, Clauss WG, Fronius M. Evidence for basolateral Cl− channels as modulators of apical Cl− secretion in pulmonary epithelia of Xenopus laevis. Am J Physiol Regul Integr Comp Physiol 300: R616–R623, 2011. doi: 10.1152/ajpregu.00464.2010. [DOI] [PubMed] [Google Scholar]
- 16.Bernard K, Bogliolo S, Soriani O, Ehrenfeld J. Modulation of calcium-dependent chloride secretion by basolateral SK4-like channels in a human bronchial cell line. J Membr Biol 196: 15–31, 2003. doi: 10.1007/s00232-003-0621-3. [DOI] [PubMed] [Google Scholar]
- 17.Birket SE, Chu KK, Houser GH, Liu L, Fernandez CM, Solomon GM, Lin V, Shastry S, Mazur M, Sloane PA, Hanes J, Grizzle WE, Sorscher EJ, Tearney GJ, Rowe SM. Combination therapy with cystic fibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am J Physiol Lung Cell Mol Physiol 310: L928–L939, 2016. doi: 10.1152/ajplung.00395.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum-Johnston C, Thorpe RB, Wee C, Romero M, Brunelle A, Blood Q, Wilson R, Blood AB, Francis M, Taylor MS, Longo LD, Pearce WJ, Wilson SM. Developmental acceleration of bradykinin-dependent relaxation by prenatal chronic hypoxia impedes normal development after birth. Am J Physiol Lung Cell Mol Physiol 310: L271–L286, 2016. doi: 10.1152/ajplung.00340.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodas M, Min T, Vij N. Critical role of CFTR-dependent lipid rafts in cigarette smoke-induced lung epithelial injury. Am J Physiol Lung Cell Mol Physiol 300: L811–L820, 2011. doi: 10.1152/ajplung.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodas M, Min T, Vij N. Critical role of CFTR dependent lipid-raft signaling in cigarette smoke induced lung injury and emphysema. Am J Respir Crit Care Med 183: A1055, 2011. doi: 10.1164/ajrccm-conference.2011.183.1_MeetingAbstracts.A1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnet S, Savineau JP, Barillot W, Dubuis E, Vandier C, Bonnet P. Role of Ca(2+)-sensitive K(+) channels in the remission phase of pulmonary hypertension in chronic obstructive pulmonary diseases. Cardiovasc Res 60: 326–336, 2003. doi: 10.1016/S0008-6363(03)00527-3. [DOI] [PubMed] [Google Scholar]
- 22.Boucher RC. Relationship of airway epithelial ion transport to chronic bronchitis. Proc Am Thorac Soc 1: 66–70, 2004. doi: 10.1513/pats.2306018. [DOI] [PubMed] [Google Scholar]
- 23.Boucher RC, Cotton CU, Gatzy JT, Knowles MR, Yankaskas JR. Evidence for reduced Cl- and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol 405: 77–103, 1988. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan SC, Wilkinson WJ, Tseng HE, Finney B, Monk B, Dibble H, Quilliam S, Warburton D, Galietta LJ, Kemp PJ, Riccardi D. The extracellular calcium-sensing receptor regulates human fetal lung development via CFTR. Sci Rep 6: 21975, 2016. doi: 10.1038/srep21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brochiero E, Dagenais A, Privé A, Berthiaume Y, Grygorczyk R. Evidence of a functional CFTR Cl− channel in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L382–L392, 2004. doi: 10.1152/ajplung.00320.2002. [DOI] [PubMed] [Google Scholar]
- 26.Brueggemann LI, Haick JM, Neuburg S, Tate S, Randhawa D, Cribbs LL, Byron KL. KCNQ (Kv7) potassium channel activators as bronchodilators: combination with a β2-adrenergic agonist enhances relaxation of rat airways. Am J Physiol Lung Cell Mol Physiol 306: L476–L486, 2014. doi: 10.1152/ajplung.00253.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brune K, Frank J, Schwingshackl A, Finigan J, Sidhaye VK. Pulmonary epithelial barrier function: some new players and mechanisms. Am J Physiol Lung Cell Mol Physiol 308: L731–L745, 2015. doi: 10.1152/ajplung.00309.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruscia EM, Zhang PX, Barone C, Scholte BJ, Homer R, Krause DS, Egan ME. Increased susceptibility of Cftr−/− mice to LPS-induced lung remodeling. Am J Physiol Lung Cell Mol Physiol 310: L711–L719, 2016. doi: 10.1152/ajplung.00284.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchanan PJ, McNally P, Harvey BJ, Urbach V. Lipoxin A4-mediated KATP potassium channel activation results in cystic fibrosis airway epithelial repair. Am J Physiol Lung Cell Mol Physiol 305: L193–L201, 2013. doi: 10.1152/ajplung.00058.2013. [DOI] [PubMed] [Google Scholar]
- 30.Buyck JM, Verriere V, Benmahdi R, Higgins G, Guery B, Matran R, Harvey BJ, Faure K, Urbach V. P. aeruginosa LPS stimulates calcium signaling and chloride secretion via CFTR in human bronchial epithelial cells. J Cyst Fibros 12: 60–67, 2013. doi: 10.1016/j.jcf.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Caci E, Scudieri P, Di Carlo E, Morelli P, Bruno S, De Fino I, Bragonzi A, Gianotti A, Sondo E, Ferrera L, Palleschi A, Santambrogio L, Ravazzolo R, Galietta LJ. Upregulation of TMEM16A protein in bronchial epithelial cells by bacterial pyocyanin. PLoS One 10: e0131775, 2015. doi: 10.1371/journal.pone.0131775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 288: L813–L819, 2005. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- 33.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 34.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 63: 152–157, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter TC, Schomberg S, Nichols C, Stenmark KR, Weil JV. Hypoxia reversibly inhibits epithelial sodium transport but does not inhibit lung ENaC or Na-K-ATPase expression. Am J Physiol Lung Cell Mol Physiol 284: L77–L83, 2003. doi: 10.1152/ajplung.00181.2002. [DOI] [PubMed] [Google Scholar]
- 36.Chauvet S, Traboulsi W, Thevenon L, Kouadri A, Feige JJ, Camara B, Alfaidy N, Benharouga M. EG-VEGF, BV8, and their receptor expression in human bronchi and their modification in cystic fibrosis: Impact of CFTR mutation (delF508). Am J Physiol Lung Cell Mol Physiol 309: L314–L322, 2015. doi: 10.1152/ajplung.00382.2014. [DOI] [PubMed] [Google Scholar]
- 37.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143: 911–923, 2010. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Fuller CM, Kleyman TR, Matalon S. Mutations in the extracellular loop of α-rENaC alter sensitivity to amiloride and reactive species. Am J Physiol Renal Physiol 286: F1202–F1208, 2004. doi: 10.1152/ajprenal.00352.2003. [DOI] [PubMed] [Google Scholar]
- 39.Chen XJ, Seth S, Yue G, Kamat P, Compans RW, Guidot D, Brown LA, Eaton DC, Jain L. Influenza virus inhibits ENaC and lung fluid clearance. Am J Physiol Lung Cell Mol Physiol 287: L366–L373, 2004. doi: 10.1152/ajplung.00011.2004. [DOI] [PubMed] [Google Scholar]
- 40.Chen XX, Zhang JH, Pan BH, Ren HL, Feng XL, Wang JL, Xiao JH. TRPC3-mediated Ca(2+) entry contributes to mouse airway smooth muscle cell proliferation induced by lipopolysaccharide. Cell Calcium 60: 273–281, 2016. doi: 10.1016/j.ceca.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z, Zhao R, Zhao M, Liang X, Bhattarai D, Dhiman R, Shetty S, Idell S, Ji HL. Regulation of epithelial sodium channels in urokinase plasminogen activator deficiency. Am J Physiol Lung Cell Mol Physiol 307: L609–L617, 2014. doi: 10.1152/ajplung.00126.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chinet TC, Gabriel SE, Penland CM, Sato M, Stutts MJ, Boucher RC, Van Scott MR. CFTR-like chloride channels in non-ciliated bronchiolar epithelial (Clara) cells. Biochem Biophys Res Commun 230: 470–475, 1997. doi: 10.1006/bbrc.1996.5939. [DOI] [PubMed] [Google Scholar]
- 43.Choi HC, Kim CS, Tarran R. Automated acquisition and analysis of airway surface liquid height by confocal microscopy. Am J Physiol Lung Cell Mol Physiol 309: L109–L118, 2015. doi: 10.1152/ajplung.00027.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chong PA, Vernon RM, Hudson R, Lin H, Forman-Kay J. How Cf mutations in Nbds affect structure and functioning of Cftr. Pediatr Pulmonol 51: 122–123, 2016. [Google Scholar]
- 45.Collawn JF, Fu L, Bartoszewski R, Matalon S. Rescuing ΔF508 CFTR with trimethylangelicin, a dual-acting corrector and potentiator. Am J Physiol Lung Cell Mol Physiol 307: L431–L434, 2014. doi: 10.1152/ajplung.00177.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 302: L1141–L1146, 2012. doi: 10.1152/ajplung.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collawn JF, Matalon S. CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol 307: L917–L923, 2014. doi: 10.1152/ajplung.00326.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coppens JT, Plopper CG, Murphy SR, Van Winkle LS. Postnatal lung development of rhesus monkey airways: cellular expression of Clara cell secretory protein. Dev Dyn 238: 3016–3024, 2009. doi: 10.1002/dvdy.22132. [DOI] [PubMed] [Google Scholar]
- 49.Crandall ED, Matthay MA. Alveolar epithelial transport. Basic science to clinical medicine. Am J Respir Crit Care Med 163: 1021–1029, 2001. doi: 10.1164/ajrccm.163.4.2006116. [DOI] [PubMed] [Google Scholar]
- 50.Crump RG, Askew GR, Wert SE, Lingrel JB, Joiner CH. In situ localization of sodium-potassium ATPase mRNA in developing mouse lung epithelium. Am J Physiol Lung Cell Mol Physiol 269: L299–L308, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Cui G, Khazanov N, Stauffer BB, Infield DT, Imhoff BR, Senderowitz H, McCarty NA. Potentiators exert distinct effects on human, murine, and Xenopus CFTR. Am J Physiol Lung Cell Mol Physiol 311: L192–L207, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui G, McCarty NA. Murine and human CFTR exhibit different sensitivities to CFTR potentiators. Am J Physiol Lung Cell Mol Physiol 309: L687–L699, 2015. doi: 10.1152/ajplung.00181.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Czikora I, Alli A, Bao HF, Kaftan D, Sridhar S, Apell HJ, Gorshkov B, White R, Zimmermann A, Wendel A, Pauly-Evers M, Hamacher J, Garcia-Gabay I, Fischer B, Verin A, Bagi Z, Pittet JF, Shabbir W, Lemmens-Gruber R, Chakraborty T, Lazrak A, Matthay MA, Eaton DC, Lucas R. A novel tumor necrosis factor-mediated mechanism of direct epithelial sodium channel activation. Am J Respir Crit Care Med 190: 522–532, 2014. doi: 10.1164/rccm.201405-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dagenais A, Fréchette R, Yamagata Y, Yamagata T, Carmel JF, Clermont ME, Brochiero E, Massé C, Berthiaume Y. Downregulation of ENaC activity and expression by TNF-α in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L301–L311, 2004. doi: 10.1152/ajplung.00326.2002. [DOI] [PubMed] [Google Scholar]
- 55.Danielsson J, Barajas M, Zhang Y, Emala C. Antagonists of The Tmem16a calcium-activated chloride channel relax airway smooth muscle and attenuate increases in intracellular calcium (Abstract). Am J Respir Crit Care Med 189: A5588, 2014. [Google Scholar]
- 56.Danielsson J, Yim P, Rinderspacher A, Fu XW, Zhang Y, Landry DW, Emala CW. Chloride channel blockade relaxes airway smooth muscle and potentiates relaxation by β-agonists. Am J Physiol Lung Cell Mol Physiol 307: L273–L282, 2014. doi: 10.1152/ajplung.00351.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 258: L134–L147, 1990. [DOI] [PubMed] [Google Scholar]
- 58.Dobbs LG, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc Natl Acad Sci USA 95: 2991–2996, 1998. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Downs CA, Helms MN. Regulation of ion transport by oxidants. Am J Physiol Lung Cell Mol Physiol 305: L595–L603, 2013. doi: 10.1152/ajplung.00212.2013. [DOI] [PubMed] [Google Scholar]
- 60.Downs CA, Kreiner L, Zhao XM, Trac P, Johnson NM, Hansen JM, Brown LA, Helms MN. Oxidized glutathione (GSSG) inhibits epithelial sodium channel activity in primary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 308: L943–L952, 2015. doi: 10.1152/ajplung.00213.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dransfield MT. Acquired Cftr dysfunction in Copd. Respirology 21: 7–7, 2016. 27005345 [Google Scholar]
- 62.Eaton AF, Yue Q, Eaton DC, Bao HF. ENaC activity and expression is decreased in the lungs of protein kinase C-α knockout mice. Am J Physiol Lung Cell Mol Physiol 307: L374–L385, 2014. doi: 10.1152/ajplung.00040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eiffler I, Behnke J, Ziesemer S, Müller C, Hildebrandt JP. Staphylococcus aureus α-toxin-mediated cation entry depolarizes membrane potential and activates p38 MAP kinase in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 311: L676–L685, 2016. doi: 10.1152/ajplung.00090.2016. [DOI] [PubMed] [Google Scholar]
- 64.Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 119: 199–207, 2002. doi: 10.1085/jgp.119.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- 66.Feraille E, Dizin E. Coordinated control of ENaC and Na+,K+-ATPase in renal collecting duct. J Am Soc Nephrol 27: 2554–2563, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer H. Function of proton channels in lung epithelia. Wiley Interdiscip Rev Membr Transp Signal 1: 247–258, 2012. doi: 10.1002/wmts.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer H, Illek B, Finkbeiner WE, Widdicombe JH. Basolateral Cl channels in primary airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol 292: L1432–L1443, 2007. doi: 10.1152/ajplung.00032.2007. [DOI] [PubMed] [Google Scholar]
- 69.Fletcher ME, Boshier PR, Wakabayashi K, Keun HC, Smolenski RT, Kirkham PA, Adcock IM, Barton PJ, Takata M, Marczin N. Influence of glutathione-S-transferase (GST) inhibition on lung epithelial cell injury: role of oxidative stress and metabolism. Am J Physiol Lung Cell Mol Physiol 308: L1274–L1285, 2015. doi: 10.1152/ajplung.00220.2014. [DOI] [PubMed] [Google Scholar]
- 70.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol 35: 10–19, 2006. doi: 10.1165/rcmb.2006-0080SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ford B. CFTR structure: lassoing cystic fibrosis. Nat Struct Mol Biol 24: 13–14, 2017. doi: 10.1038/nsmb.3353. [DOI] [PubMed] [Google Scholar]
- 72.Ford B, Collins RF, Meng X. G551d Cftr 3d structure determined by cryo-electron microscopy. Pediatr Pulmonol 51: 195–196, 2016. [Google Scholar]
- 73.Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, Matthay MA, Pittet JF. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 278: 43939–43950, 2003. doi: 10.1074/jbc.M304882200. [DOI] [PubMed] [Google Scholar]
- 74.Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med 2: a009563, 2012. doi: 10.1101/cshperspect.a009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu L, Rab A, Tang L, Bebok Z, Rowe SM, Bartoszewski R, Collawn JF. ΔF508 CFTR surface stability is regulated by DAB2 and CHIP-mediated ubiquitination in post-endocytic compartments. PLoS One 10: e0123131, 2015. doi: 10.1371/journal.pone.0123131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fukuda N, Jayr C, Lazrak A, Wang Y, Lucas R, Matalon S, Matthay MA. Mechanisms of TNF-α stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 280: L1258–L1265, 2001. [DOI] [PubMed] [Google Scholar]
- 77.Galam L, Rajan A, Failla A, Soundararajan R, Lockey RF, Kolliputi N. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression. Am J Physiol Lung Cell Mol Physiol 310: L572–L581, 2016. doi: 10.1152/ajplung.00417.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galietta LJV, Pagesy P, Folli C, Caci E, Romio L, Costes B, Nicolis E, Cabrini G, Goossens M, Ravazzolo R, Zegarra-Moran O. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J Immunol 168: 839–845, 2002. doi: 10.4049/jimmunol.168.2.839. [DOI] [PubMed] [Google Scholar]
- 79.Gallos G, Remy KE, Danielsson J, Funayama H, Fu XW, Chang HY, Yim P, Xu D, Emala CW Sr. Functional expression of the TMEM16 family of calcium-activated chloride channels in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 305: L625–L634, 2013. doi: 10.1152/ajplung.00068.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gallos G, Yocum GT, Siviski ME, Yim PD, Fu XW, Poe MM, Cook JM, Harrison N, Perez-Zoghbi J, Emala CW Sr. Selective targeting of the α5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am J Physiol Lung Cell Mol Physiol 308: L931–L942, 2015. doi: 10.1152/ajplung.00107.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garantziotis S, Brezina M, Castelnuovo P, Drago L. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am J Physiol Lung Cell Mol Physiol 310: L785–L795, 2016. doi: 10.1152/ajplung.00168.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia-Caballero A, Rasmussen JE, Gaillard E, Watson MJ, Olsen JC, Donaldson SH, Stutts MJ, Tarran R. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci USA 106: 11412–11417, 2009. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaunsbaek MQ, Kjeldsen AD, Svane-Knudsen V, Henriksen ML, Hansen S. Surfactant proteins A, B, C and D in the human nasal airway: associated with mucosal glands and ciliated epithelium but absent in fluid-phase secretions and mucus. ORL J Otorhinolaryngol Relat Spec 76: 288–301, 2014. doi: 10.1159/000369143. [DOI] [PubMed] [Google Scholar]
- 84.Gentzsch M, Ren HY, Houck SA, Quinney NL, Cholon DM, Sopha P, Chaudhry IG, Das J, Dokholyan NV, Randell SH, Cyr DM. Restoration of R117H CFTR folding and function in human airway cells through combination treatment with VX-809 and VX-770. Am J Physiol Lung Cell Mol Physiol 311: L550–L559, 2016. doi: 10.1152/ajplung.00186.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilbert G, Ducret T, Savineau JP, Marthan R, Quignard JF. Caveolae are involved in mechanotransduction during pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L1078–L1087, 2016. doi: 10.1152/ajplung.00198.2015. [DOI] [PubMed] [Google Scholar]
- 86.Giraldez T, Afonso-Oramas D, Cruz-Muros I, Garcia-Marin V, Pagel P, González-Hernández T, Alvarez de la Rosa D. Cloning and functional expression of a new epithelial sodium channel delta subunit isoform differentially expressed in neurons of the human and monkey telencephalon. J Neurochem 102: 1304–1315, 2007. doi: 10.1111/j.1471-4159.2007.04622.x. [DOI] [PubMed] [Google Scholar]
- 87.Giraldez T, Rojas P, Jou J, Flores C, Alvarez de la Rosa D. The epithelial sodium channel δ-subunit: new notes for an old song. Am J Physiol Renal Physiol 303: F328–F338, 2012. doi: 10.1152/ajprenal.00116.2012. [DOI] [PubMed] [Google Scholar]
- 88.Gonzalez Bosc LV, Plomaritas DR, Herbert LM, Giermakowska W, Browning C, Jernigan NL. ASIC1-mediated calcium entry stimulates NFATc3 nuclear translocation via PICK1 coupling in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 311: L48–L58, 2016. doi: 10.1152/ajplung.00040.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T, Dard N, Planès C, Matthay MA, Clerici C. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol 306: L975–L985, 2014. doi: 10.1152/ajplung.00242.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Greenlee MM, Mitzelfelt JD, Yu L, Yue Q, Duke BJ, Harrell CS, Neigh GN, Eaton DC. Estradiol activates epithelial sodium channels in rat alveolar cells through the G protein-coupled estrogen receptor. Am J Physiol Lung Cell Mol Physiol 305: L878–L889, 2013. doi: 10.1152/ajplung.00008.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gregory DJ, Kobzik L. Influenza lung injury: mechanisms and therapeutic opportunities. Am J Physiol Lung Cell Mol Physiol 309: L1041–L1046, 2015. doi: 10.1152/ajplung.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grzesik B, Gabrielli NM, Fronius M, Morty RE, Seeger W, Sznajder JI, Vadasz I. Long-term hypercapnia impairs ENaC function in alveolar epithelial monolayers: a role for ubiquitination? Am J Respir Crit Care Med 183: A4228, 2011. doi: 10.1164/ajrccm-conference.2011.183.1_MeetingAbstracts.A4228. [DOI] [Google Scholar]
- 93.Guggino S. Cyclic nucleotide-gated cation channels contribute to sodium absorption in lung: role of nonselective cation channels. Curr Topic Membr 47: 279–294, 1999. doi: 10.1016/S0070-2161(08)60964-8. [DOI] [Google Scholar]
- 94.Gwoździńska P, Buchbinder BA, Mayer K, Herold S, Morty RE, Seeger W, Vadász I. Hypercapnia impairs ENaC cell surface stability by promoting phosphorylation, polyubiquitination and endocytosis of β-ENaC in a human alveolar epithelial cell line. Front Immunol 8: 591, 2017. doi: 10.3389/fimmu.2017.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gwozdzinska P, Buchbinder BA, Mazzocchi LC, Seeger W, Vadasz I. Hypercapnia impairs enac stability in primary alveolar epithelial cells (Abstract). Am J Respir Crit Care Med 191: A2034, 2015. [Google Scholar]
- 96.Haas M, Forbush B III. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol 62: 515–534, 2000. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 97.Haick JM, Brueggemann LI, Cribbs LL, Denning MF, Schwartz J, Byron KL. PKC-dependent regulation of Kv7.5 channels by the bronchoconstrictor histamine in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 312: L822–L834, 2017. doi: 10.1152/ajplung.00567.2016. [DOI] [PubMed] [Google Scholar]
- 98.Hardiman KM, Matalon S. Modification of sodium transport and alveolar fluid clearance by hypoxia: mechanisms and physiological implications. Am J Respir Cell Mol Biol 25: 538–541, 2001. doi: 10.1165/ajrcmb.25.5.f219. [DOI] [PubMed] [Google Scholar]
- 99.Hendrick SM, Mroz MS, Greene CM, Keely SJ, Harvey BJ. Bile acids stimulate chloride secretion through CFTR and calcium-activated Cl− channels in Calu-3 airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 307: L407–L418, 2014. doi: 10.1152/ajplung.00352.2013. [DOI] [PubMed] [Google Scholar]
- 100.Henry CO, Dalloneau E, Pérez-Berezo MT, Plata C, Wu Y, Guillon A, Morello E, Aimar RF, Potier-Cartereau M, Esnard F, Coraux C, Börnchen C, Kiefmann R, Vandier C, Touqui L, Valverde MA, Cenac N, Si-Tahar M. In vitro and in vivo evidence for an inflammatory role of the calcium channel TRPV4 in lung epithelium: Potential involvement in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 311: L664–L675, 2016. doi: 10.1152/ajplung.00442.2015. [DOI] [PubMed] [Google Scholar]
- 101.Hobbs CA, Blanchard MG, Alijevic O, Tan CD, Kellenberger S, Bencharit S, Cao R, Kesimer M, Walton WG, Henderson AG, Redinbo MR, Stutts MJ, Tarran R. Identification of the SPLUNC1 ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol 305: L990–L1001, 2013. doi: 10.1152/ajplung.00103.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hofer CC, Woods PS, Davis IC. Infection of mice with influenza A/WSN/33 (H1N1) virus alters alveolar type II cell phenotype. Am J Physiol Lung Cell Mol Physiol 308: L628–L638, 2015. doi: 10.1152/ajplung.00373.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hollenhorst MI, Richter K, Fronius M. Ion transport by pulmonary epithelia. J Biomed Biotechnol 2011: 174306, 2011. doi: 10.1155/2011/174306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD, Donne ML, Huang X, Sheppard D, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M, Jan YN, Jan LY, Rock JR. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci USA 109: 16354–16359, 2012. doi: 10.1073/pnas.1214596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ilumets H, Mazur W, Toljamo T, Louhelainen N, Nieminen P, Kobayashi H, Ishikawa N, Kinnula VL. Ageing and smoking contribute to plasma surfactant proteins and protease imbalance with correlations to airway obstruction. BMC Pulm Med 11: 19, 2011. doi: 10.1186/1471-2466-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Infield DT, Cui G, Kuang C, McCarty NA. Positioning of extracellular loop 1 affects pore gating of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Lung Cell Mol Physiol 310: L403–L414, 2016. doi: 10.1152/ajplung.00259.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ismailov II, Awayda MS, Jovov B, Berdiev BK, Fuller CM, Dedman JR, Kaetzel M, Benos DJ. Regulation of epithelial sodium channels by the cystic fibrosis transmembrane conductance regulator. J Biol Chem 271: 4725–4732, 1996. doi: 10.1074/jbc.271.9.4725. [DOI] [PubMed] [Google Scholar]
- 108.Itani OA, Auerbach SD, Husted RF, Volk KA, Ageloff S, Knepper MA, Stokes JB, Thomas CP. Glucocorticoid-stimulated lung epithelial Na+ transport is associated with regulated ENaC and sgk1 expression. Am J Physiol Lung Cell Mol Physiol 282: L631–L641, 2002. doi: 10.1152/ajplung.00085.2001. [DOI] [PubMed] [Google Scholar]
- 109.Ito Y, Correll K, Zemans RL, Leslie CC, Murphy RC, Mason RJ. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-α/EGFR signaling. Am J Physiol Lung Cell Mol Physiol 308: L1178–L1188, 2015. doi: 10.1152/ajplung.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ji HL, Zhao RZ, Chen ZX, Shetty S, Idell S, Matalon S. δ-ENaC: a novel divergent amiloride-inhibitable sodium channel. Am J Physiol Lung Cell Mol Physiol 303: L1013–L1026, 2012. doi: 10.1152/ajplung.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jia J, Conlon TM, Ballester Lopez C, Seimetz M, Bednorz M, Zhou-Suckow Z, Weissmann N, Eickelberg O, Mall MA, Yildirim AO. Cigarette smoke causes acute airway disease and exacerbates chronic obstructive lung disease in neonatal mice. Am J Physiol Lung Cell Mol Physiol 311: L602–L610, 2016. doi: 10.1152/ajplung.00124.2016. [DOI] [PubMed] [Google Scholar]
- 112.Jiang X, Ingbar DH, O’Grady SM. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl− channels. Am J Physiol Cell Physiol 275: C1610–C1620, 1998. [DOI] [PubMed] [Google Scholar]
- 113.Jin N, Kolliputi N, Gou D, Weng T, Liu L. A novel function of ionotropic gamma-aminobutyric acid receptors involving alveolar fluid homeostasis. J Biol Chem 281: 36012–36020, 2006. doi: 10.1074/jbc.M606895200. [DOI] [PubMed] [Google Scholar]
- 114.Jin NL, Kolliputi N, Gou DM, Weng TT, Liu L. A novel fluid transport pathway in adult rat alveolar epithelial type II cells. FASEB J 20: A745, 2006. doi: 10.1096/fj.1530-6860. [DOI] [Google Scholar]
- 115.Johnson M, Allen L, Dobbs L. Characteristics of Cl− uptake in rat alveolar type I cells. Am J Physiol Lung Cell Mol Physiol 297: L816–L827, 2009. doi: 10.1152/ajplung.90466.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 103: 4964–4969, 2006. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci USA 99: 1966–1971, 2002. doi: 10.1073/pnas.042689399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jovanović S, Crawford RM, Ranki HJ, Jovanović A. Large conductance Ca2+-activated K+ channels sense acute changes in oxygen tension in alveolar epithelial cells. Am J Respir Cell Mol Biol 28: 363–372, 2003. doi: 10.1165/rcmb.2002-0101OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Junor RW, Benjamin AR, Alexandrou D, Guggino SE, Walters DV. Lack of a role for cyclic nucleotide gated cation channels in lung liquid absorption in fetal sheep. J Physiol 523: 493–502, 2000. doi: 10.1111/j.1469-7793.2000.t01-3-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Junor RW, Benjamin AR, Alexandrou D, Guggino SE, Walters DV. A novel role for cyclic nucleotide-gated cation channels in lung liquid homeostasis in sheep. J Physiol 520: 255–260, 1999. doi: 10.1111/j.1469-7793.1999.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kalinowski L, Janaszak-Jasiecka A, Siekierzycka A, Bartoszewska S, Woźniak M, Lejnowski D, Collawn JF, Bartoszewski R. Posttranscriptional and transcriptional regulation of endothelial nitric-oxide synthase during hypoxia: the role of microRNAs. Cell Mol Biol Lett 21: 16, 2016. doi: 10.1186/s11658-016-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kelly O, Wang J, Chen L, Matalon S, Ma HP. Ceramide mediates cytokine-induced pulmonary edema by inhibiting ENaC. FASEB J 20: A1231, 2006. doi: 10.1096/fj.1530-6860. [DOI] [Google Scholar]
- 123.Kemp PJ, Kim KJ, Borok Z, Crandall ED. Re-evaluating the Na(+) conductance of adult rat alveolar type II pneumocytes: evidence for the involvement of cGMP-activated cation channels. J Physiol 536: 693–701, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kemp PJ, Lewis A, Hartness ME, Searle GJ, Miller P, O’Kelly I, Peers C. Airway chemotransduction: from oxygen sensor to cellular effector. Am J Respir Crit Care Med 166, Suppl 1: S17–S24, 2002. doi: 10.1164/rccm.2206009. [DOI] [PubMed] [Google Scholar]
- 125.Kim C, Tan C, Gilmore R, Walton W, Redinbo M, Tarran R. Mechanistic insight into short palate lung and nasal epithelial clone 1 (splunc1)-epithelial sodium channel (enac) interactions in cystic fibrosis epithelia. Am J Respir Crit Care Med 189: A2370, 2014. [Google Scholar]
- 126.Kim CS, Tarran R. Short palate lung and nasal epithelial clone 1 (Splunc1) dissociates and internalizes the epithelial sodium channel (Enac). Pediatr Pulmonol 50: 228–229, 2015. [Google Scholar]
- 127.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kulaksiz H, Schmid A, Hönscheid M, Ramaswamy A, Cetin Y. Clara cell impact in air-side activation of CFTR in small pulmonary airways. Proc Natl Acad Sci USA 99: 6796–6801, 2002. doi: 10.1073/pnas.102171199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kunzelmann K, Kathöfer S, Greger R. Na+ and Cl- conductances in airway epithelial cells: increased Na+ conductance in cystic fibrosis. Pflugers Arch 431: 1–9, 1995. doi: 10.1007/BF00374371. [DOI] [PubMed] [Google Scholar]
- 130.Kunzelmann K, Pavenstädt H, Beck C, Unal O, Emmrich P, Arndt HJ, Greger R. Characterization of potassium channels in respiratory cells. I. General properties. Pflugers Arch 414: 291–296, 1989. doi: 10.1007/BF00584629. [DOI] [PubMed] [Google Scholar]
- 131.Kunzelmann K, Schreiber R, Nitschke R, Mall M. Control of epithelial Na+ conductance by the cystic fibrosis transmembrane conductance regulator. Pflugers Arch 440: 193–201, 2000. doi: 10.1007/s004240000255. [DOI] [PubMed] [Google Scholar]
- 132.Lakunina VA, Burnysheva KM, Mitkevich VA, Makarov AA, Petrushanko IY. [Changes in the receptor function of Na,K-ATPase during hypoxia and ischemia]. Mol Biol (Mosk) 51: 172–179, 2017. doi: 10.1134/S0026893317010101. [DOI] [PubMed] [Google Scholar]
- 133.Lang JD, McArdle PJ, O’Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest Supplt 122: 314S–320S, 2002. doi: 10.1378/chest.122.6_suppl.314S. [DOI] [PubMed] [Google Scholar]
- 134.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: L557–L567, 2011. doi: 10.1152/ajplung.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lazrak A, Jurkuvenaite A, Ness EC, Zhang S, Woodworth BA, Muhlebach MS, Stober VP, Lim YP, Garantziotis S, Matalon S. Inter-α-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in ΔF508 mice. Am J Respir Cell Mol Biol 50: 953–962, 2014. doi: 10.1165/rcmb.2013-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lazrak A, Matalon S. cAMP-induced changes of apical membrane potentials of confluent H441 monolayers. Am J Physiol Lung Cell Mol Physiol 285: L443–L450, 2003. doi: 10.1152/ajplung.00412.2002. [DOI] [PubMed] [Google Scholar]
- 138.Lecuona E, Trejo HE, Sznajder JI. Regulation of Na,K-ATPase during acute lung injury. J Bioenerg Biomembr 39: 391–395, 2007. doi: 10.1007/s10863-007-9102-1. [DOI] [PubMed] [Google Scholar]
- 139.Leroy C, Privé A, Bourret JC, Berthiaume Y, Ferraro P, Brochiero E. Regulation of ENaC and CFTR expression with K+ channel modulators and effect on fluid absorption across alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L1207–L1219, 2006. doi: 10.1152/ajplung.00376.2005. [DOI] [PubMed] [Google Scholar]
- 140.Liu F, Zhang Z, Csanady L, Gadsby DC, Chen J. Molecular structure of the human CFTR ion channel. Cell 169: 85–95, 2017. doi: 10.1016/j.cell.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 141.Livraghi-Butrico A, Kelly EJ, Wilkinson KJ, Rogers TD, Gilmore RC, Harkema JR, Randell SH, Boucher RC, O’Neal WK, Grubb BR. Loss of Cftr function exacerbates the phenotype of Na+ hyperabsorption in murine airways. Am J Physiol Lung Cell Mol Physiol 304: L469–L480, 2013. doi: 10.1152/ajplung.00150.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol 35: 116–129, 2007. doi: 10.1080/01926230601060025. [DOI] [PubMed] [Google Scholar]
- 143.Londino JD, Lazrak A, Collawn JF, Bebok Z, Harrod KS, Matalon S. Influenza virus infection alters ion channel function of airway and alveolar cells: mechanisms and physiological sequelae. Am J Physiol Lung Cell Mol Physiol 00244 02017, 2017. doi: 10.1152/ajplung.00244.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Londino JD, Lazrak A, Jurkuvenaite A, Collawn JF, Noah JW, Matalon S. Influenza matrix protein 2 alters CFTR expression and function through its ion channel activity. Am J Physiol Lung Cell Mol Physiol 304: L582–L592, 2013. doi: 10.1152/ajplung.00314.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Londino JD, Lazrak A, Noah JW, Aggarwal S, Bali V, Woodworth BA, Bebok Z, Matalon S. Influenza virus M2 targets cystic fibrosis transmembrane conductance regulator for lysosomal degradation during viral infection. FASEB J 29: 2712–2725, 2015. doi: 10.1096/fj.14-268755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lucas R, Yue Q, Alli A, Duke BJ, Al-Khalili O, Thai TL, Hamacher J, Sridhar S, Lebedyeva I, Su H, Tzotzos S, Fischer B, Gameiro AF, Loose M, Chakraborty T, Shabbir W, Aufy M, Lemmens-Gruber R, Eaton DC, Czikora I. The Lectin-like Domain of TNF Increases ENaC open probability through a novel site at the interface between the second transmembrane and C-terminal domains of the α-subunit. J Biol Chem 291: 23440–23451, 2016. doi: 10.1074/jbc.M116.718163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lv Y, Tang LL, Wei JK, Xu XF, Gu W, Fu LC, Zhang LY, Du LZ. Decreased Kv1.5 expression in intrauterine growth retardation rats with exaggerated pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 305: L856–L865, 2013. doi: 10.1152/ajplung.00179.2013. [DOI] [PubMed] [Google Scholar]
- 148.Malerba M, Radaeli A, Mancuso S, Polosa R. The potential therapeutic role of potassium channel modulators in asthma and chronic obstructive pulmonary disease. J Biol Regul Homeost Agents 24: 123–130, 2010. [PubMed] [Google Scholar]
- 149.Mall M, Gonska T, Thomas J, Schreiber R, Seydewitz HH, Kuehr J, Brandis M, Kunzelmann K. Modulation of Ca2+-activated Cl- secretion by basolateral K+ channels in human normal and cystic fibrosis airway epithelia. Pediatr Res 53: 608–618, 2003. doi: 10.1203/01.PDR.0000057204.51420.DC. [DOI] [PubMed] [Google Scholar]
- 150.Mall M, Wissner A, Schreiber R, Kuehr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Role of K(V)LQT1 in cyclic adenosine monophosphate-mediated Cl(-) secretion in human airway epithelia. Am J Respir Cell Mol Biol 23: 283–289, 2000. doi: 10.1165/ajrcmb.23.3.4060. [DOI] [PubMed] [Google Scholar]
- 151.Mandegar M, Yuan JX. Role of K+ channels in pulmonary hypertension. Vascul Pharmacol 38: 25–33, 2002. doi: 10.1016/S1537-1891(02)00123-4. [DOI] [PubMed] [Google Scholar]
- 152.Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, Larsson HP, Salathe M. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem 286: 19830–19839, 2011. doi: 10.1074/jbc.M110.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Manzanares D, Srinivasan M, Salathe ST, Ivonnet P, Baumlin N, Dennis JS, Conner GE, Salathe M. IFN-γ-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. Am J Physiol Lung Cell Mol Physiol 306: L453–L462, 2014. doi: 10.1152/ajplung.00247.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol 103: 1649–1656, 1991. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Matalon S. Mechanisms and regulation of ion transport in adult mammalian alveolar type II pneumocytes. Am J Physiol Cell Physiol 261: C727–C738, 1991. [DOI] [PubMed] [Google Scholar]
- 156.Matalon S, Bartoszewski R, Collawn JF. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol 309: L1229–L1238, 2015. doi: 10.1152/ajplung.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Matalon S, O’Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 61: 627–661, 1999. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- 158.Matsui H, Davis CW, Tarran R, Boucher RC. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J Clin Invest 105: 1419–1427, 2000. doi: 10.1172/JCI4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Matthay MA, Clerici C, Saumon G. Active fluid clearance from the distal air spaces of the lung. J Appl Physiol (1985) 93: 1533–1541, 2002. doi: 10.1152/japplphysiol.01210.2001. [DOI] [PubMed] [Google Scholar]
- 160.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 161.Matthay MA, Landolt CC, Staub NC. Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol Respir Environ Exerc Physiol 53: 96–104, 1982. [DOI] [PubMed] [Google Scholar]
- 162.Migneault F, Boncoeur E, Morneau F, Pascariu M, Dagenais A, Berthiaume Y. Cycloheximide and lipopolysaccharide downregulate αENaC mRNA via different mechanisms in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 305: L747–L755, 2013. doi: 10.1152/ajplung.00023.2013. [DOI] [PubMed] [Google Scholar]
- 163.Molina SA, Stauffer B, Moriarty HK, Kim AH, McCarty NA, Koval M. Junctional abnormalities in human airway epithelial cells expressing F508del CFTR. Am J Physiol Lung Cell Mol Physiol 309: L475–L487, 2015. doi: 10.1152/ajplung.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Morty RE, Kuebler WM. TRPV4: An exciting new target to promote alveolocapillary barrier function. Am J Physiol Lung Cell Mol Physiol 307: L817–L821, 2014. doi: 10.1152/ajplung.00254.2014. [DOI] [PubMed] [Google Scholar]
- 165.Moudgil R, Michelakis ED, Archer SL. The role of k+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation 13: 615–632, 2006. doi: 10.1080/10739680600930222. [DOI] [PubMed] [Google Scholar]
- 166.Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, Matalon S, Hollenberg S, Factor P. Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling. Circ Res 94: 1091–1100, 2004. doi: 10.1161/01.RES.0000125623.56442.20. [DOI] [PubMed] [Google Scholar]
- 167.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem 286: 2365–2374, 2011. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 169.O’Dwyer DN, Ashley SL, Moore BB. Influences of innate immunity, autophagy, and fibroblast activation in the pathogenesis of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 311: L590–L601, 2016. doi: 10.1152/ajplung.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.O’Grady SM, Lee SY. Chloride and potassium channel function in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 284: L689–L700, 2003. doi: 10.1152/ajplung.00256.2002. [DOI] [PubMed] [Google Scholar]
- 171.O’Grady SM, Lee SY. Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol 37: 1578–1594, 2005. doi: 10.1016/j.biocel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 172.Oh Y, Hu P, Kleyman TR, Saccomani G, Matalon S, Benos DJ. Evidence for the presence of an amiloride binding-protein in adult alveolar type-Ii (Atii) pneumocytes. FASEB J 5: A690, 1991. [PubMed] [Google Scholar]
- 173.Orgeig S, McGillick EV, Botting KJ, Zhang S, McMillen IC, Morrison JL. Increased lung prolyl hydroxylase and decreased glucocorticoid receptor are related to decreased surfactant protein in the growth-restricted sheep fetus. Am J Physiol Lung Cell Mol Physiol 309: L84–L97, 2015. doi: 10.1152/ajplung.00275.2014. [DOI] [PubMed] [Google Scholar]
- 174.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 373: 1891–1904, 2009. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 175.Ouellet C, Bilodeau G, Cantin AM. Oxidative stress, smoking and CFTR: can cystic fibrosis be acquired? Med Sci (Paris) 23: 9–10, 2007. doi: 10.1051/medsci/20072319. [DOI] [PubMed] [Google Scholar]
- 176.Paradiso AM. ATP-activated basolateral Na+/H+ exchange in human normal and cystic fibrosis airway epithelium. Am J Physiol Lung Cell Mol Physiol 273: L148–L158, 1997. [DOI] [PubMed] [Google Scholar]
- 177.Paradiso AM. Identification of Na+-H+ exchange in human normal and cystic fibrotic ciliated airway epithelium. Am J Physiol Lung Cell Mol Physiol 262: L757–L764, 1992. [DOI] [PubMed] [Google Scholar]
- 178.Park HS, Kim SR, Lee YC. Impact of oxidative stress on lung diseases. Respirology 14: 27–38, 2009. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 179.Peitzman ER, Zaidman NA, Maniak PJ, O’Grady SM. Carvedilol binding to β2-adrenergic receptors inhibits CFTR-dependent anion secretion in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 310: L50–L58, 2016. doi: 10.1152/ajplung.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Peters DM, Vadász I, Wujak L, Wygrecka M, Olschewski A, Becker C, Herold S, Papp R, Mayer K, Rummel S, Brandes RP, Günther A, Waldegger S, Eickelberg O, Seeger W, Morty RE. TGF-β directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc Natl Acad Sci USA 111: E374–E383, 2014. doi: 10.1073/pnas.1306798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Planès C, Friedlander G, Loiseau A, Amiel C, Clerici C. Inhibition of Na-K-ATPase activity after prolonged hypoxia in an alveolar epithelial cell line. Am J Physiol Lung Cell Mol Physiol 271: L70–L78, 1996. [DOI] [PubMed] [Google Scholar]
- 182.Plomaritas DR, Herbert LM, Yellowhair TR, Resta TC, Gonzalez Bosc LV, Walker BR, Jernigan NL. Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L419–L430, 2014. doi: 10.1152/ajplung.00095.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372: 415–417, 2008. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 184.Rab A, Rowe SM, Raju SV, Bebok Z, Matalon S, Collawn JF. Cigarette smoke and CFTR: implications in the pathogenesis of COPD. Am J Physiol Lung Cell Mol Physiol 305: L530–L541, 2013. doi: 10.1152/ajplung.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 186.Rahman M, Mukherjee S, Sheng W, Nilius B, Janssen LJ. Electrophysiological characterization of voltage-dependent calcium currents and TRPV4 currents in human pulmonary fibroblasts. Am J Physiol Lung Cell Mol Physiol 310: L603–L614, 2016. doi: 10.1152/ajplung.00426.2015. [DOI] [PubMed] [Google Scholar]
- 187.Raju SV, Trombley J, Lin V, Kim H, Tang LP, Samuel S, Kumar R, Winter L, Morrow C, Schoeb T, Rowe S. Acquired Cftr dysfunction and chronic bronchitis in a novel ferret model of Copd. FASEB J 191: A3865, 2015. [Google Scholar]
- 188.Rauh R, Soell D, Haerteis S, Diakov A, Nesterov V, Krueger B, Sticht H, Korbmacher C. A mutation in the β-subunit of ENaC identified in a patient with cystic fibrosis-like symptoms has a gain-of-function effect. Am J Physiol Lung Cell Mol Physiol 304: L43–L55, 2013. doi: 10.1152/ajplung.00093.2012. [DOI] [PubMed] [Google Scholar]
- 189.Reilly EC, Martin KC, Jin GB, Yee M, O’Reilly MA, Lawrence BP. Neonatal hyperoxia leads to persistent alterations in NK responses to influenza A virus infection. Am J Physiol Lung Cell Mol Physiol 308: L76–L85, 2015. doi: 10.1152/ajplung.00233.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Remillard CV, Yuan JX. High altitude pulmonary hypertension: role of K+ and Ca2+ channels. High Alt Med Biol 6: 133–146, 2005. doi: 10.1089/ham.2005.6.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rock JR, O’Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl- secretory channel in mouse airways. J Biol Chem 284: 14875–14880, 2009. doi: 10.1074/jbc.C109.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem 280: 18579–18589, 2005. doi: 10.1074/jbc.M410561200. [DOI] [PubMed] [Google Scholar]
- 193.Rowe SM, Dransfield MT. Lower airway potential difference measurements in copd subjects: emerging evidence of acquired Cftr dysfunction. Pediatr Pulmonol 216–217, 2010. [Google Scholar]
- 194.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 195.Sabusap CM, Wang W, McNicholas CM, Chung WJ, Fu L, Wen H, Mazur M, Kirk KL, Collawn JF, Hong JS, Sorscher EJ. Analysis of cystic fibrosis-associated P67L CFTR illustrates barriers to personalized therapeutics for orphan diseases. JCI Insight 1: e86581, 2016. doi: 10.1172/jci.insight.86581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Salomon JJ, Spahn S, Wang X, Füllekrug J, Bertrand CA, Mall MA. Generation and functional characterization of epithelial cells with stable expression of SLC26A9 Cl− channels. Am J Physiol Lung Cell Mol Physiol 310: L593–L602, 2016. doi: 10.1152/ajplung.00321.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Saumon G, Basset G. Electrolyte and fluid transport across the mature alveolar epithelium. J Appl Physiol (1985) 74: 1–15, 1993. [DOI] [PubMed] [Google Scholar]
- 198.Schiffhauer ES, Vij N, Kovbasnjuk O, Kang PW, Walker D, Lee S, Zeitlin PL. Dual activation of CFTR and CLCN2 by lubiprostone in murine nasal epithelia. Am J Physiol Lung Cell Mol Physiol 304: L324–L331, 2013. doi: 10.1152/ajplung.00277.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schmiedl A, Krainski J, Schwichtenhövel F, Schade J, Klemann C, Raber KA, Zscheppang K, Beekmann T, Acevedo C, Glaab T, Wedekind D, Pabst R, von Hörsten S, Stephan M. Reduced airway inflammation in CD26/DPP4-deficient F344 rats is associated with altered recruitment patterns of regulatory T cells and expression of pulmonary surfactant proteins. Clin Exp Allergy 40: 1794–1808, 2010. doi: 10.1111/j.1365-2222.2010.03547.x. [DOI] [PubMed] [Google Scholar]
- 200.Schreiber R, Uliyakina I, Kongsuphol P, Warth R, Mirza M, Martins JR, Kunzelmann K. Expression and function of epithelial anoctamins. J Biol Chem 285: 7838–7845, 2010. doi: 10.1074/jbc.M109.065367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res 569: 29–63, 2005. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 202.Schroeder TH, Lee MM, Yacono PW, Cannon CL, Gerçeker AA, Golan DE, Pier GB. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc Natl Acad Sci USA 99: 6907–6912, 2002. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schwarzer GC, Kim E, Fischer H, Gruenert D, Ames BN, Peden DB, Machen T, Illek B. Dual effects of pyocyanin-induced oxidative stress on CFTR chloride transport (Abstract). Pediatr Pulmonol 251–252, 2007. [Google Scholar]
- 204.Schwiebert EM, Cid-Soto LP, Stafford D, Carter M, Blaisdell CJ, Zeitlin PL, Guggino WB, Cutting GR. Analysis of ClC-2 channels as an alternative pathway for chloride conduction in cystic fibrosis airway cells. Proc Natl Acad Sci USA 95: 3879–3884, 1998. doi: 10.1073/pnas.95.7.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schwingshackl A. The role of stretch-activated ion channels in acute respiratory distress syndrome: finally a new target? Am J Physiol Lung Cell Mol Physiol 311: L639–L652, 2016. doi: 10.1152/ajplung.00458.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schwingshackl A, Bryan R, Lloyd E, Makena P, Teng B, Ghosh M, Sinclair S, Waters C. Deficiency of the two-pore-domain potassium channel Trek-1 promotes hyperoxia-induced lung injury. Crit Care Med 41: A44–A45, 2013. doi: 10.1097/01.ccm.0000439348.75835.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schwingshackl A, Teng B, Ghosh M, Lim K, Tigyi G, Narayanan D, Jaggar J, Waters C. The two-pore-domain potassium (k2p) channel Trek-1 regulates Il-6 release from alveolar epithelial cells independently of intra-and extracellular Ca2+. Am J Respir Crit Care Med 187: A1886, 2013. [Google Scholar]
- 208.Schwingshackl A, Teng B, Ghosh M, Lim KG, Tigyi G, Narayanan D, Jaggar JH, Waters CM. Regulation of interleukin-6 secretion by the two-pore-domain potassium channel Trek-1 in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 304: L276–L286, 2013. doi: 10.1152/ajplung.00299.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sedivy V, Joshi S, Ghaly Y, Mizera R, Zaloudikova M, Brennan S, Novotna J, Herget J, Gurney AM. Role of Kv7 channels in responses of the pulmonary circulation to hypoxia. Am J Physiol Lung Cell Mol Physiol 308: L48–L57, 2015. doi: 10.1152/ajplung.00362.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sharma P, Panebra A, Pera T, Tiegs BC, Hershfeld A, Kenyon LC, Deshpande DA. Antimitogenic effect of bitter taste receptor agonists on airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 310: L365–L376, 2016. doi: 10.1152/ajplung.00373.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shijubo N, Itoh Y, Yamaguchi T, Imada A, Hirasawa M, Yamada T, Kawai T, Abe S. Clara cell protein-positive epithelial cells are reduced in small airways of asthmatics. Am J Respir Crit Care Med 160: 930–933, 1999. doi: 10.1164/ajrccm.160.3.9803113. [DOI] [PubMed] [Google Scholar]
- 212.Simard CF, Bergeron MJ, Frenette-Cotton R, Carpentier GA, Pelchat ME, Caron L, Isenring P. Homooligomeric and heterooligomeric associations between K+-Cl- cotransporter isoforms and between K+-Cl- and Na+-K+-Cl- cotransporters. J Biol Chem 282: 18083–18093, 2007. doi: 10.1074/jbc.M607811200. [DOI] [PubMed] [Google Scholar]
- 213.Skou JC. The identification of the sodium pump. Biosci Rep 18: 155–169, 1998. doi: 10.1023/A:1020196612909. [DOI] [PubMed] [Google Scholar]
- 214.Sommer D, Bogdan R, Berger J, Peters DM, Morty RE, Clauss WG, Fronius M. CFTR-dependent Cl- secretion in Xenopus laevis lung epithelium. Respir Physiol Neurobiol 158: 97–106, 2007. doi: 10.1016/j.resp.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 215.Sondo E, Pesce E, Tomati V, Marini M, Pedemonte N. RNF5, DAB2 and friends: novel drug targets for cystic fibrosis. Curr Pharm Des 23: 176–186, 2017. doi: 10.2174/1381612822666161006161033. [DOI] [PubMed] [Google Scholar]
- 216.Song T, Hao Q, Zheng YM, Liu QH, Wang YX. Inositol 1,4,5-trisphosphate activates TRPC3 channels to cause extracellular Ca2+ influx in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 309: L1455–L1466, 2015. doi: 10.1152/ajplung.00148.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Song Y, Namkung W, Nielson DW, Lee JW, Finkbeiner WE, Verkman AS. Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: Dependence on Na+ and Cl− channel function. Am J Physiol Lung Cell Mol Physiol 297: L1131–L1140, 2009. doi: 10.1152/ajplung.00085.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Spahis S, Ntimbane T, Mailhot G, Kleme ML, Berthiaume Y, Rabassa-Lhoret R, Levy E. Impact of CFTR on insulin secretion, oxidative stress and inflammation in cystic fibrosis-related diabetes. Gastroenterology 146: S497–S498, 2014. doi: 10.1016/S0016-5085(14)61795-9. [DOI] [Google Scholar]
- 219.Spina D. Epithelium smooth muscle regulation and interactions. Am J Respir Crit Care Med 158, Suppl 2: S141–S145, 1998. doi: 10.1164/ajrccm.158.supplement_2.13tac100a. [DOI] [PubMed] [Google Scholar]
- 220.Suresh K, Rentsendorj O, Undem C, Shimoda LA, Servinsky LE, Willette RN, Pearse DB. TRPV4 activation increases endothelial permeability and [Ca2+](i) in human lung microvascular endothelial cells. Am J Respir Crit Care Med 181: A3425, 2010. doi: 10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A3425. [DOI] [Google Scholar]
- 221.Suresh K, Servinsky L, Reyes J, Baksh S, Undem C, Caterina M, Pearse DB, Shimoda LA. Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves TRPV4. Am J Physiol Lung Cell Mol Physiol 309: L1467–L1477, 2015. doi: 10.1152/ajplung.00275.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Suresh K, Servinsky L, Reyes J, Undem C, Zaldumbide J, Rentsendorj O, Modekurty S, Dodd-O JM, Scott A, Pearse DB, Shimoda LA. CD36 mediates H2O2-induced calcium influx in lung microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 312: L143–L153, 2017. doi: 10.1152/ajplung.00361.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Suresh K, Shimoda L, Pearse DB. Ros-induced calcium influx in human lung microvascular endothelial cells involves Trpv4. Am J Respir Crit Care Med 191: A2664, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sznajder JI, Factor P, Ingbar DH. Invited review: lung edema clearance: role of Na+-K+-ATPase. J Appl Physiol (1985) 93: 1860–1866, 2002. doi: 10.1152/japplphysiol.00022.2002. [DOI] [PubMed] [Google Scholar]
- 225.Tafel O, Latzin P, Paul K, Winter T, Woischnik M, Griese M. Surfactant proteins SP-B and SP-C and their precursors in bronchoalveolar lavages from children with acute and chronic inflammatory airway disease. BMC Pulm Med 8: 6, 2008. doi: 10.1186/1471-2466-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Takemura Y, Helms MN, Eaton AF, Self J, Ramosevac S, Jain L, Bao HF, Eaton DC. Cholinergic regulation of epithelial sodium channels in rat alveolar type 2 epithelial cells. Am J Physiol Lung Cell Mol Physiol 304: L428–L437, 2013. doi: 10.1152/ajplung.00129.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tarran R, Sabater JR, Clarke TC, Tan CD, Davies CM, Liu J, Yeung A, Garland AL, Stutts MJ, Abraham WM, Phillips G, Baker WR, Wright CD, Wilbert S. Nonantibiotic macrolides prevent human neutrophil elastase-induced mucus stasis and airway surface liquid volume depletion. Am J Physiol Lung Cell Mol Physiol 304: L746–L756, 2013. doi: 10.1152/ajplung.00292.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tessier GJ, Traynor TR, Kannan MS, O’Grady SM. Mechanisms of sodium and chloride transport across equine tracheal epithelium. Am J PhysioPhysiol Lung Cell Mol Physiol 259: L459–L467, 1990. [DOI] [PubMed] [Google Scholar]
- 229.Thannickal VJ, Day RM, Klinz SG, Bastien MC, Larios JM, Fanburg BL. Ras-dependent and -independent regulation of reactive oxygen species by mitogenic growth factors and TGF-beta1. FASEB J 14: 1741–1748, 2000. doi: 10.1096/fj.99-0878com. [DOI] [PubMed] [Google Scholar]
- 230.Thome U, Chen L, Factor P, Dumasius V, Freeman B, Sznajder JI, Matalon S. Na,K-ATPase gene transfer mitigates an oxidant-induced decrease of active sodium transport in rat fetal ATII cells. Am J Respir Cell Mol Biol 24: 245–252, 2001. doi: 10.1165/ajrcmb.24.3.3994. [DOI] [PubMed] [Google Scholar]
- 231.Thome UH, Nguyen SV, Ware J, Matalon S. Effects of oxygen tension and corticosterone on sodium transport in rat fetal alveolar cells. FASEB J 15: A859, 2001. [Google Scholar]
- 232.Thompson-Vest N, Shimizu Y, Hunne B, Furness JB. The distribution of intermediate-conductance, calcium-activated, potassium (IK) channels in epithelial cells. J Anat 208: 219–229, 2006. doi: 10.1111/j.1469-7580.2006.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Trinh NT, Privé A, Kheir L, Bourret JC, Hijazi T, Amraei MG, Noël J, Brochiero E. Involvement of KATP and KvLQT1 K+ channels in EGF-stimulated alveolar epithelial cell repair processes. Am J Physiol Lung Cell Mol Physiol 293: L870–L882, 2007. doi: 10.1152/ajplung.00362.2006. [DOI] [PubMed] [Google Scholar]
- 234.Vadász I, Raviv S, Sznajder JI. Alveolar epithelium and Na,K-ATPase in acute lung injury. Intensive Care Med 33: 1243–1251, 2007. doi: 10.1007/s00134-007-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vohwinkel CU, Vadász I. Influenza A matrix protein M2 downregulates CFTR: Inhibition of chloride transport by a proton channel of the viral envelope. Am J Physiol Lung Cell Mol Physiol 304: L813–L816, 2013. doi: 10.1152/ajplung.00091.2013. [DOI] [PubMed] [Google Scholar]
- 236.Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem 270: 27411–27414, 1995. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- 237.Wan J, Yamamura A, Zimnicka AM, Voiriot G, Smith KA, Tang H, Ayon RJ, Choudhury MS, Ko EA, Wang J, Wang C, Makino A, Yuan JX. Chronic hypoxia selectively enhances L- and T-type voltage-dependent Ca2+ channel activity in pulmonary artery by upregulating Cav1.2 and Cav3.2. Am J Physiol Lung Cell Mol Physiol 305: L154–L164, 2013. doi: 10.1152/ajplung.00313.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Weidenfeld S, Kuebler WM. Cytokine-regulation of Na(+)-K(+)-Cl(-) cotransporter 1 and cystic fibrosis transmembrane conductance regulator-potential role in pulmonary inflammation and edema formation. Front Immunol 8: 393, 2017. doi: 10.3389/fimmu.2017.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wilkinson WJ, Benjamin AR, De Proost I, Orogo-Wenn MC, Yamazaki Y, Staub O, Morita T, Adriaensen D, Riccardi D, Walters DV, Kemp PJ. Alveolar epithelial CNGA1 channels mediate cGMP-stimulated, amiloride-insensitive, lung liquid absorption. Pflugers Arch 462: 267–279, 2011. doi: 10.1007/s00424-011-0971-0. [DOI] [PubMed] [Google Scholar]
- 240.Wolk KE, Lazarowski ER, Traylor ZP, Yu EN, Jewell NA, Durbin RK, Durbin JE, Davis IC. Influenza A virus inhibits alveolar fluid clearance in BALB/c mice. Am J Respir Crit Care Med 178: 969–976, 2008. doi: 10.1164/rccm.200803-455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wu H, Yang J, Su EM, Li L, Zhao C, Yang X, Gao Z, Pan M, Sun P, Sun W, Jiang Y, Su X. Lipoxin A4 and platelet activating factor are involved in E. coli or LPS-induced lung inflammation in CFTR-deficient mice. PLoS One 9: e93003, 2014. doi: 10.1371/journal.pone.0093003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wu T, Walton W, Redinbo M, Alexis NE, Tarran R. Splunc1 modulates calcium influx in airway smooth muscle. Am J Respir Crit Care Med 193: A4642, 2016. [Google Scholar]
- 243.Yue G, Shoemaker RL, Matalon S. Regulation of low-amiloride-affinity sodium channels in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 267: L94–L100, 1994. [DOI] [PubMed] [Google Scholar]
- 244.Zaidi T, Mowrey-McKee M, Pier GB. Hypoxia increases corneal cell expression of CFTR leading to increased Pseudomonas aeruginosa binding, internalization, and initiation of inflammation. Invest Ophthalmol Vis Sci 45: 4066–4074, 2004. doi: 10.1167/iovs.04-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zdebik AA, Cuffe JE, Bertog M, Korbmacher C, Jentsch TJ. Additional disruption of the ClC-2 Cl(-) channel does not exacerbate the cystic fibrosis phenotype of cystic fibrosis transmembrane conductance regulator mouse models. J Biol Chem 279: 22276–22283, 2004. doi: 10.1074/jbc.M309899200. [DOI] [PubMed] [Google Scholar]
- 246.Zeitlin PL. Cystic fibrosis and estrogens: a perfect storm. J Clin Invest 118: 3841–3844, 2008. doi: 10.1172/JCI37778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang Z, Chen J. Atomic structure of the cystic fibrosis transmembrane conductance regulator. Cell 167: 1586–1597, 2016. doi: 10.1016/j.cell.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 248.Zhang Z, Leir SH, Harris A. Oxidative stress regulates CFTR gene expression in human airway epithelial cells through a distal antioxidant response element. Am J Respir Cell Mol Biol 52: 387–396, 2015. doi: 10.1165/rcmb.2014-0263OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhao R, Liang X, Zhao M, Liu SL, Huang Y, Idell S, Li X, Ji HL. Correlation of apical fluid-regulating channel proteins with lung function in human COPD lungs. PLoS One 9: e109725, 2014. doi: 10.1371/journal.pone.0109725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zheng YM, Wang YX. Molecular mechanism and functional role of Trpc3 channels in asthmatic airway hyperresponsiveness and remodeling. Am J Respir Crit Care Med 193: A2842, 2016. [Google Scholar]
- 251.Zhou C, Townsley MI, Alexeyev M, Voelkel NF, Stevens T. Endothelial hyperpermeability in severe pulmonary arterial hypertension: role of store-operated calcium entry. Am J Physiol Lung Cell Mol Physiol 311: L560–L569, 2016. doi: 10.1152/ajplung.00057.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhuang J, Zhao L, Zang N, Xu F. Prenatal nicotinic exposure augments cardiorespiratory responses to activation of bronchopulmonary C-fibers. Am J Physiol Lung Cell Mol Physiol 308: L922–L930, 2015. doi: 10.1152/ajplung.00241.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]