Abstract
The prevalence, presenting clinical and pathological characteristics, and outcomes for patients with diffuse large B-cell lymphoma that is Epstein-Barr virus positive remain uncertain as does the impact of congenital or iatrogenic immunosuppression. Patients with newly diagnosed diffuse large B-cell lymphoma with available tissue arrays were identified from the University of Iowa/Mayo Clinic Molecular Epidemiology Resource. Patients infected with human immunodeficiency virus or who had undergone a prior organ transplant were excluded. Epstein-Barr virus-associated ribonucleic acid testing was performed on all tissue arrays. A history of significant congenital or iatrogenic immunosuppression was determined for all patients. At enrollment, 16 of the 362 (4.4%) biopsies were positive for Epstein-Barr virus. Thirty-nine (10.8%) patients had a significant history of immunosuppression. Patients with Epstein-Barr-positive diffuse large B-cell lymphoma had no unique clinical characteristics but on pathology exhibited a higher frequency of CD30 positivity (25.0% versus 8.1%, respectively; P<0.01), and non-germinal-center subtype (62.5% versus 34.1%, respectively; P<0.01). No baseline clinical characteristics were associated with a history of immunosuppression. With a median follow up of 59 months, and after adjustment for International Prognostic Index, there was no association of Epstein-Barr virus positivity or immunosuppression with event-free survival at 24 months (odds ratio=0.49; 95% confidence interval: 0.13–1.84 and odds ratio=0.81; 95% confidence interval: 0.37–1.77) or overall survival (hazard ratio=0.86; 95% confidence interval: 0.38–1.97 and hazard ratio=1.00; 95% confidence interval: 0.57–1.74). In contrast to non-Western populations, our North American population had a low prevalence of Epstein-Barr virus-positive diffuse large B-cell lymphoma that did not convey an adverse prognosis. A history of immunosuppression, while known to be a risk factor for the development of diffuse large B-cell lymphoma, did not affect subsequent prognosis.
Introduction
Diffuse large B-cell lymphoma (DLBCL) displays substantial clinical and pathological heterogeneity. Epstein-Barr virus (EBV)-positive DLBCL of the elderly (EDLBCLe) was a provisional entity in the 2008 World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues1 defined as an EBV+ clonal B-cell lymphoid proliferation that occurs in patients older than 50 years, without any known immunodeficiency or prior lymphoma. EDLBCLe has been found to account for 2–16% of all DLBCL in Asia or Latin countries, with most studies’ estimates at the higher end of this range.2–7 Such reports generally describe an association of EBV positivity with advanced stage, extranodal disease, constitutional symptoms and low rates of response to chemotherapy, with resultant poor survival.4–6,8 In contrast, studies focused on patients in Western countries have generally found both lower prevalence rates and weaker associations of EBV with aggressive clinical features or inferior outcomes.9–11 Direct comparison of these studies is limited by their inclusion of selected cases rather than prospective cohorts. Furthermore, multiple studies from both Western and non-Western populations have now also identified EBV+ DLBCL in immunocompetent younger patients, in addition to elderly ones.4,8 The 2016 WHO classification was revised to recognize the wide age spectrum of patients affected by this condition, and “EBV+ DLBCL of the elderly” has been replaced by “EBV+ DLBCL, not otherwise specified” (EDLBCL-NOS).12,13
A further area of uncertainty concerns the relative impact of EBV positivity versus immunosuppression itself on adverse outcomes among patients with DLBCL. EBV+ lymphomas arise more frequently in patients with compromised immune systems, as exemplified in EBV+ post-transplant lymphoproliferative disease. However, immunosuppression due to iatrogenic agents or congenital immunodeficiency has also been shown to be a risk factor for EBV− DLBCL.14,15 More broadly, it is unknown whether immunosuppression is also associated with poor outcomes among DLBCL patients in general.
In light of these considerable uncertainties, we sought to define the prevalence, clinical correlations, and prognosis of EBV+ DLBCL among a prospectively assembled cohort of patients from the upper Midwestern area of the USA. To delineate the effects of immune dysfunction from those of EBV, standardized definitions were developed for clinically significant immunosuppression, and a subgroup analysis of immunocompetent and immunosuppressed patients was performed. This study represents a large systematic predominantly prospective evaluation of EDLB-CL-NOS in the USA, as well as the first independent examination of the effects of immune suppression on outcomes among patients with DLBCL.
Methods
Study population
This study was approved by institutional review boards at the University of Iowa and Mayo Clinic. Written informed consent was obtained from all participants. This study utilized the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence which has been previously described.16,17 Briefly, starting from September 2002, we offered enrollment to consecutive, newly diagnosed adult patients with lymphoma evaluated at the University of Iowa or Mayo Clinic Rochester. All diagnoses were confirmed by study hematopathologists. Diagnostic tissue blocks from DLBCL cases were collected and tissue microarrays were constructed using three 0.6 mm cores from all cases with sufficient tissue to be used in subsequent bulk analyses. Baseline clinical, laboratory, and treatment data were collected. All participants were systematically contacted for follow-up every 6 months for the first 3 years, and then annually thereafter. Disease progression, retreatment, and death were verified through review of medical records. Inclusion criteria for this analysis were initial diagnosis of de novo or transformed DLBCL enrolled from 2002–2012, with tissue microarrays. Patients with a primary central nervous system lymphoma, primary cutaneous lymphoma, or primary mediastinal large B-cell lymphoma were excluded, as were patients with a history of organ transplant or known infection with human immunodeficiency virus.
Immunohistochemistry and in situ hybridization
Immunohistochemistry for CD30, CD10, bcl-6, and MUM1 was centrally scored on tissue microarrays. Cell of origin was determined according to the Hans criteria.18 The cutoff for CD30 positivity was 20% of neoplastic cells.19 EBV testing was performed by in situ hybridization for EBER with a threshold of 30% of neoplastic cells, and scored by an expert hematopathologist (AD, AF).20
Definition of immunosuppression or immunodeficiency
The prospectively collected information was augmented for this analysis with a retrospective chart review focused on evidence of immunosuppression for each patient included. One patient with a history of congenital immunodeficiency was identified. Patients with a documented history of prior treatment with methotrexate, cyclophosphamide, azathioprine, hydroxychloroquine, antiepileptic agents, or biologic agents including anti-tumor necrosis factor monoclonal antibodies were considered to have received iatrogenic immunosuppression, as were patients who had received a lifetime exposure to corticosteroids equal to or greater than 6 months of daily prednisone at a dose of 20 mg/day. Patients with a seizure history treated with antiepileptic drugs were also considered immunodeficient based on papers describing quantitative and qualitative defects in circulating lymphocytes associated with antiepileptic therapy.21–23
Statistical analysis
EBV and immunosuppression status were correlated with clinical features using Wilcoxon signed-rank and chi-square testing, where appropriate. Events were defined as documented DLBCL progression, subsequent anti-lymphoma therapy, or death. Overall survival was defined as time from diagnosis to death. EFS24 was defined as event-free survival status at 24 months after diagnosis.24 Survival estimates were obtained with the Kaplan– Meier method. Survival comparisons between and within positive, negative, and indeterminate groupings as well as International Prognostic Index (IPI) groupings were performed with log-rank tests. The associations of EBV and immunosuppression history with event-free and overall survival were estimated using hazard ratios (HR) and 95% confidence intervals (CI) for Cox models, adjusted for IPI; logistic regression and odds ratios (OR) were used for associations with EFS24. All tests were two-sided and assessed for significance at the 5% level.
Results
Patients’ characteristics, prevalence estimates and clinical correlates
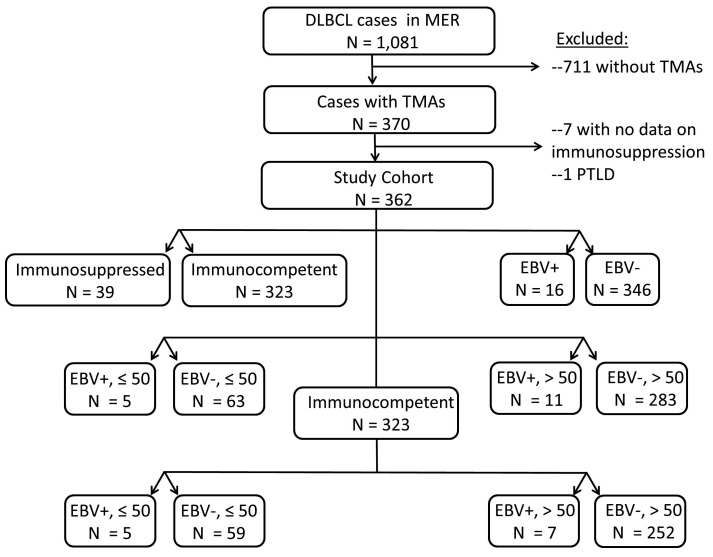
From 2002 through 2012, 1,081 patients with DLBCL were enrolled into the Molecular Epidemiology Resource, of whom 362 had diagnostic tissue on an available tissue microarray, met the study selection criteria, and form the population analyzed for this report (Figure 1). As compared to patients enrolled in the Molecular Epidemiology Resource who did not have sufficient tissue available for tissue microarray construction, study cohort patients had lower Ann Arbor stages, lactate dehydrogenase values, IPI scores, longer follow-up, and more often achieved EFS24 (Online Supplementary Table S1). Treatment regimens utilized for the DLBCL patients were largely anthracycline-based immunochemotherapy as described in more detail elsewhere.17
Figure 1.
CONSORT diagram of patients included in the study.
The median age at enrollment of the cohort analyzed was 63 years (range, 20–89) and 59% were male. EBV testing was positive in 16 (4.4%) of the cases. The baseline characteristics by tissue EBV positivity are detailed in Table 1. Fifteen of the 16 EBV+ patients were treated initially with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and one with etoposide and procarbazine substituting for doxorubicin and vincristine (R-CEPP). The age and gender distributions, performance status, Ann Arbor Stage, IPI scores, numbers of affected extranodal sites, and lactate dehydrogenase levels of the EBV− cases were similar to those of the EBV+ cases. Bone marrow involvement with large-cell lymphoma was more common among patients with EBV+ disease (43.8% versus 18.5%; P=0.03). Non-germinal center B-cell subtype disease was more frequent among EBV+ DLBCL than among EBV− DLBCL (62.5% versus 34.1%; P<0.01). CD30 positivity was also more frequent among EBV+ cases (25.0% versus 8.1%; P<0.01).
Table 1.
Patients’ demographics and clinical characteristics.
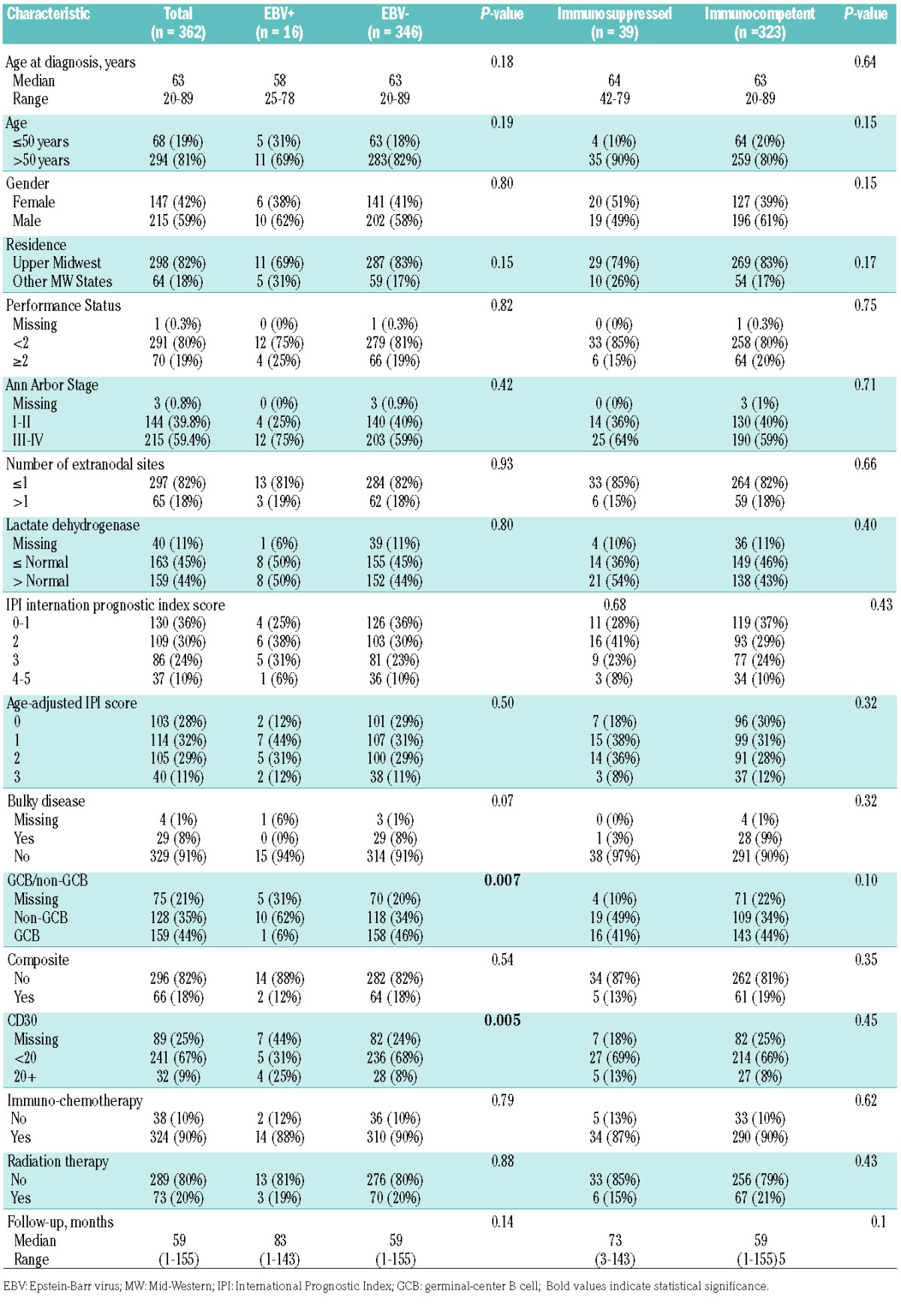
Including all patients regardless of EBV tumor status, 39 (10.8%) of the analysis cohort were considered immunosuppressed following our systematic review. Of these 39 patients 38 had received significant iatrogenic immunosuppression – though none with rituximab, while one patient had a history of common variable immunodeficiency; Online Supplementary Table S2 details the types of immunosuppressive agents administered, as well as their indications. There were no differences in baseline characteristics between immunocompetent versus immunosuppressed patients (Table 1). The management of immunosuppressive agents after the diagnosis of DLBCL was highly variable.
Seven of the 362 DLBCL cases met the 2008 WHO criteria for EDLBCLe (EBV+, age >50 years at the time of diagnosis, and immunocompetent), a prevalence rate of 1.9%. Twelve of the 362 cases met the 2016 WHO criteria for EBV+ DLBCL-NOS (EBV+, immunocompetent), a prevalence rate of 4.4%.
Prognosis
During a median follow-up of 59 months (range, 0.7–155.2), there were 150 events including 119 deaths. The median overall survival for all 362 patients was 11.7 years.
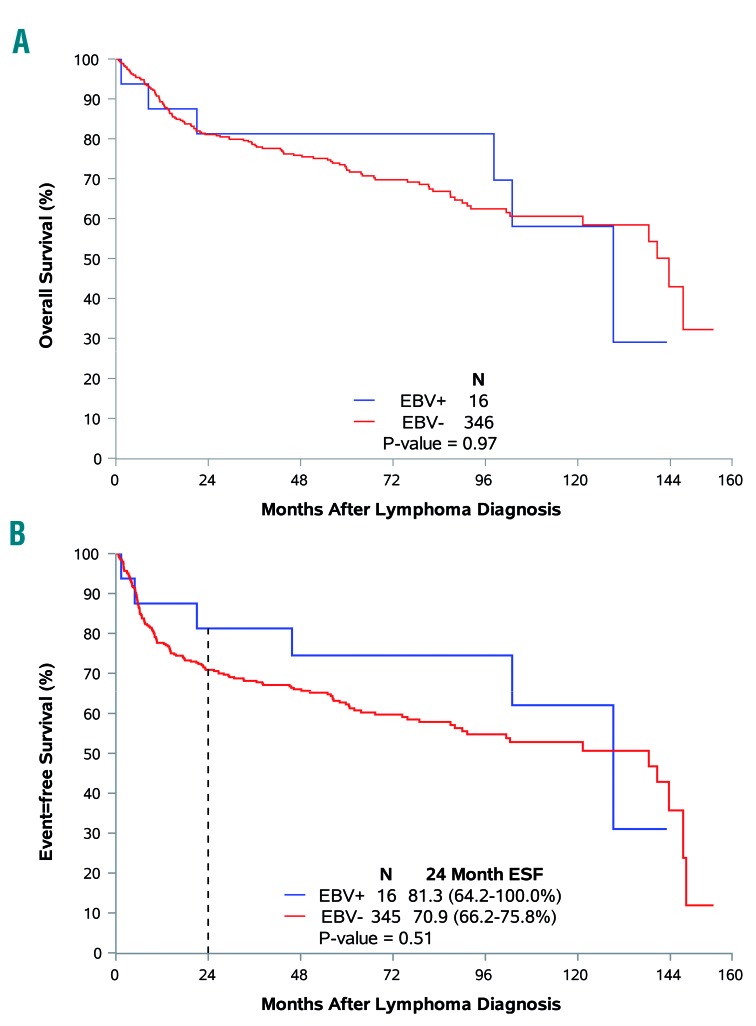
Analyzing the influence of EBV status, it was seen that the median overall survival was similar in patients with EBV+ or EBV− DLBCL (129 versus 143 months, respectively; P=0.97). EBV+ status was not associated with adverse overall survival (HR adjusted for IPI=0.86; 95% CI: 0.38–1.97) (Figure 2A). The median event-free survival was also similar in EBV+ and EBV− DLBCL patients (129 versus 138 months, P=0.51; HR adjusted for IPI =0.64; 95% CI; 0.28–1.46) (Figure 2D). Kaplan-Meier estimates for the percentage of patients reaching EFS24 were 81% for EBV+ patients, and 71% for EBV− patients (P=0.52; OR adjusted for IPI=0.49; 95% CI: 0.13–1.84).
Figure 2.
Association of Epstein-Barr virus tumor positivity with clinical endpoints. (A) Tumor EBV status and overall survival. (B) Tumor EBV status and event-free survival.
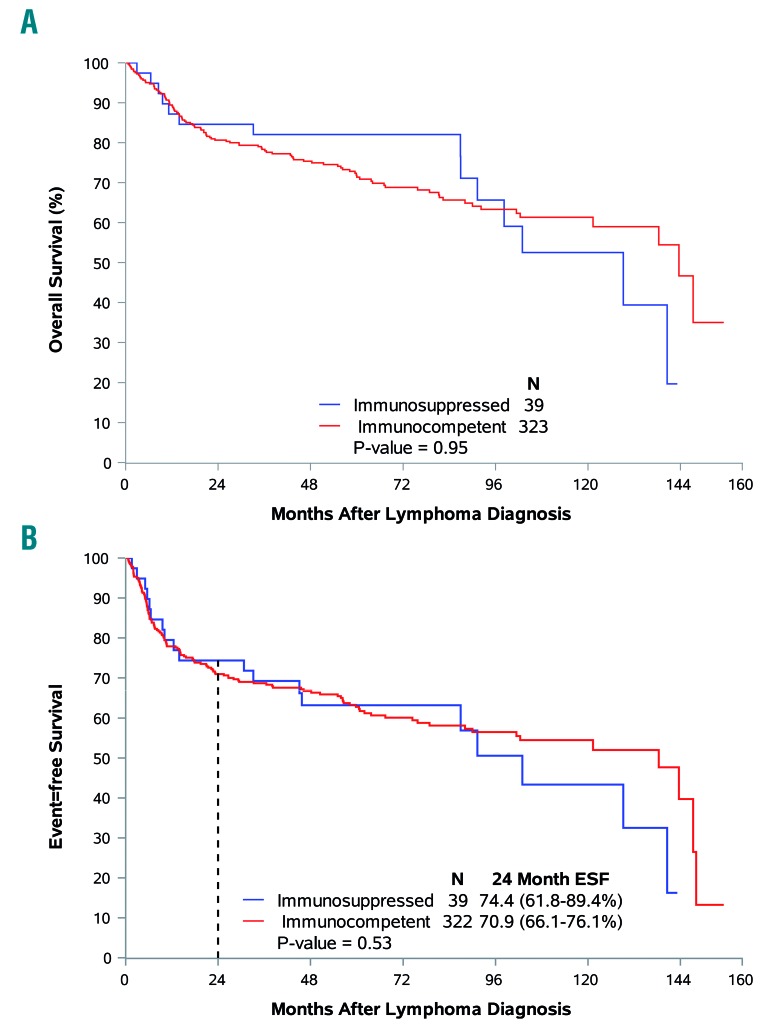
In order to evaluate the prognostic importance of immunosuppression, outcomes were compared between the 323 immunocompetent patients and 39 patients with a history of immunosuppression. Outcomes were not affected by a history of immunosuppression with the median overall survival being 129 months for patients with a history of immunosuppression versus 143 months for immunocompetent patients (P=0.95; HR adjusted for IPI=1.00; 95% CI: 0.57–1.74) and the median event-free survival being 103 months versus 138 months, respectively (HR adjusted for IPI=1.09; 95% CI: 0.67–1.76) (Figure 3). Kaplan-Meier estimates for the percentage of patients reaching EFS24 were 71% for immunocompetent patients versus 74% for patients with a history of immunosuppression (P=0.53; OR adjusted for IPI= 0.81; 95% CI: 0.37–1.77).
Figure 3.
Association of immunosuppression with clinical endpoints. (A) Immune status and overall survival. (B) Immune status and event-free survival.
Discussion
We describe one of the largest systematic evaluations of EBV+ DLBCL in a North American human immunodeficiency virus-negative population in the modern immunochemotherapy era, and the first using 2016 updated WHO definitions of EBV+ DLBCL-NOS. As a means of distinguishing the relative prognostic importance of immunosuppression from that of EBV positivity, we additionally report a survival analysis of patients with DLBCL occurring on a background of significant iatrogenic immune suppression or congenital immunodeficiency. Overall, EBV+ DLBCL occurred infrequently within the studied population. EBV+ DLBCL was found in patients of all age groups. In contrast to multiple reports focused on Asian or Latin patients, we did not observe an association of EBV positivity with aggressive presentation or adverse prognosis. Finally, while immunosuppression is a known risk factor for the development of both EBV+ and EBV– DLBCL, there was no evidence that immunosuppression was independently associated with adverse outcomes.
The prevalence of EBV+ DLBCL varies across geographical regions. Estimates of the prevalence of EDLBCLe among patients from Asian or Latin countries range from 2–12%. In contrast, a previous screening study in the USA identified five selected patients with EBV+ DLBCL, but found no further cases in screening of 90 unselected older patients with DLBCL.25 Ok et al., in a recent study of 732 patients of all age groups with DLBCL from developed Western countries, identified 28 (4.0%) as EBV+.26 Our prevalence estimates of 4.4% for EBV+ DLBCL-NOS, and 1.9% for EDLBCLe, are generally in agreement with prior reports from Western countries.
Previous series have described distinct clinical and pathological characteristics associated with EBV positivity.5–7,10–14 In agreement with prior reports, we found an association of EBV positivity with the non-germinal center B-cell subtype of DLBCL, as well as with CD30 positivity.26 However, we did not find evidence of an association of EBV positivity with aggressive clinical features such as B symptoms, multiple sites of extranodal involvement, or older age.
Several reports focused on Asian and Latin patients have suggested an association of EBV positivity with an overall worse clinical prognosis. Older large Korean and Peruvian series demonstrated inferior outcomes for EBV+ DLBCL patients.5,27 In the immunochemotherapy era, at least two subsequent studies continued to demonstrate inferior outcomes among Asian patients treated with R-CHOP.28,29 Our findings are more consistent with those of a previous study of patients from Western populations, which found no evidence of an association of EBV positivity with adverse outcomes.26 The numbers of EBV+ DLBCL patients and events included in our study were low and, accordingly, the results should be considered more descriptive than conclusive, but comparable to those of other studies which demonstrated significant differences in outcomes, suggesting adequate power.8,28
To date, no consensus definition has been adopted by the WHO to define significant clinical immunosuppression, leading to the variable inclusion of patients with a history of immunocompromised states in previous studies of EBV+ DLBCL. This suggested a possible confounding of immunosuppression with EBV status in prior reports associating EBV positivity with poor outcomes. To explore this hypothesis, we developed standard definitions of clinically significant immunosuppression, and used them to identify such patients in our cohort. Direct comparison of immunosuppressed and immunocompetent DLBCL patients revealed similar outcomes, and subgroup analysis of immunocompetent EBV+ versus EBV− DLBCL patients also found no difference. We conclude that among predominantly white North American patients, a history of immunosuppression is unlikely to confer an adverse prognosis. These results require verification in subsequent cohorts, using standardized definitions of immunosuppression.
Strengths of this study include the prospective cohort design of consecutively enrolled, newly diagnosed lymphoma patients; central pathology review; systematically collected clinical data; virtually complete follow-up of the cohort for disease progression and death; and medical record validation of these events. Our series of 362 systematically studied patients with biopsy material is one of the largest cohorts from the Western hemisphere published to date, and all patients were managed in the current immunochemotherapy era. Limitations include the modest and imperfectly representative availability of biopsy material sufficient for tissue microarray production (362 of 1,081), and the modest number of EBV+ patients which limits the power of any subset analyses. Although prospectively assembled, some analyses were retrospective in nature with all incumbent limitations. Finally, we were unable to quantify the degree of immunocompetence satisfactorily. Clearly a remote history of a brief course of immunosuppression is very different from ongoing aggressive immunosuppression, but given the subjectivity of comparing one immunosuppressing regimen to another and the complexity of factoring host issues, we chose a dichotomous “any” versus “none” variable. Over 85% of those identified as receiving immunosuppressive therapy were on such at the time of diagnosis. Attempts at quantifying immunosuppression could be the target of future research.
In summary, we found that EBV+ DLBCL represents a small fraction of DLBCL. Outcomes among EBV+ DLBCL patients are not significantly worse than those among their EBV− counterparts. The lack of difference in clinical outcomes between the studied subsets should suggest to practicing hematologists that prognosis is independent of EBV status or a history of immunosuppression, among North American patients.
Supplementary Material
Acknowledgments
We thank Julianne Lunde, Laura Jacobus, Ashley McCarthy and the study staff at Mayo Clinic Rochester and University of Iowa. This work was supported by grants from the National Institutes of Health (Lymphoma SPORE (P50 CA CA97274), Lymphoma Epidemiology of Outcomes (U01 CA195568) and the Predolin Foundation.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/2/297
Presented in abstract form at the 58th annual meeting of the American Society of Hematology, San Diego, CA December 3rd 2016
References
- 1.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn JS, Yang DH, Duk Choi Y, et al. Clinical outcome of elderly patients with Epstein-Barr virus positive diffuse large B-cell lymphoma treated with a combination of rituximab and CHOP chemotherapy. Am J Hematol. 2013;88(9):774–779. [DOI] [PubMed] [Google Scholar]
- 3.Beltran BE, Castillo JJ, Morales D, et al. EBV-positive diffuse large B-cell lymphoma of the elderly: a case series from Peru. Am J Hematol. 2011;86(8):663–667. [DOI] [PubMed] [Google Scholar]
- 4.Hong JY, Yoon DH, Suh C, et al. EBV-positive diffuse large B-cell lymphoma in young adults: is this a distinct disease entity? Ann Oncol. 2015;26(3):548–555. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Lee J, Ko YH, et al. The impact of Epstein-Barr virus status on clinical outcome in diffuse large B-cell lymphoma. Blood. 2007;110(3):972–978. [DOI] [PubMed] [Google Scholar]
- 6.Sato A, Nakamura N, Kojima M, et al. Clinical outcome of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly in the rituximab era. Cancer Sci. 2014;105(9):1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada N, Ikeda J, Hori Y, et al. Epstein-Barr virus in diffuse large B-cell lymphoma in immunocompetent patients in Japan is as low as in Western countries. J Med Virol. 2011;83(2):317–321. [DOI] [PubMed] [Google Scholar]
- 8.Lu TX, Liang JH, Miao Y, et al. Epstein-Barr virus positive diffuse large B-cell lymphoma predict poor outcome, regardless of the age. Sci Rep. 2015;5:1216–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen M, Narbaitz M, Metrebian F, De Matteo E, Preciado MV, Chabay PA. Epstein-Barr virus-positive diffuse large B-cell lymphoma association is not only restricted to elderly patients. Int J Cancer. 2014;135(12):2816–2824. [DOI] [PubMed] [Google Scholar]
- 10.Hoeller S, Tzankov A, Pileri SA, Went P, Dirnhofer S. Epstein-Barr virus-positive diffuse large B-cell lymphoma in elderly patients is rare in Western populations. Hum Pathol. 2010;41(3):352–357. [DOI] [PubMed] [Google Scholar]
- 11.Ok CY, Li L, Xu-Monette ZY, et al. Prevalence and clinical implications of epstein-barr virus infection in de novo diffuse large B-cell lymphoma in Western countries. Clin Can Res. 2014;20(9):2338–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo JJ, Beltran BE, Miranda RN, Young KH, Chavez JC, Sotomayor EM. EBV-positive diffuse large B-cell lymphoma of the elderly: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(5):529–537. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kersey JH, Shapiro RS, Filipovich AH. Relationship of immunodeficiency to lymphoid malignancy. Pediatr Infect Dis J. 1988;7(5 Suppl):S10–12. [PubMed] [Google Scholar]
- 15.Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):405–408. [DOI] [PubMed] [Google Scholar]
- 16.Drake MT, Maurer MJ, Link BK, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(27):4191–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerhan JR, Link BK, Habermann TM, et al. Cohort Profile: The Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) cohort study. Int J Epidemiol. 2017;46(6): 1753–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. [DOI] [PubMed] [Google Scholar]
- 19.Hu S, Xu-Monette ZY, Balasubramanyam A, et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2013;121(14):2715–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuhlmann-Laeisz C, Szczepanowski M, Borchert A, Bruggemann M, Klapper W. Epstein-Barr virus-negative diffuse large B-cell lymphoma hosts intra- and peritumoral B-cells with activated Epstein-Barr virus. Virchows Arch. 2015;466(1):85–92. [DOI] [PubMed] [Google Scholar]
- 21.Dinopoulos A, Attilakos A, Paschalidou M, et al. Short-term effect of levetiracetam monotherapy on haematological parameters in children with epilepsy: a prospective study. Epilepsy Res. 2014;108(4):820–823. [DOI] [PubMed] [Google Scholar]
- 22.Guenther S, Bauer S, Hagge M, et al. Chronic valproate or levetiracetam treatment does not influence cytokine levels in humans. Seizure. 2014;23(8):666–669. [DOI] [PubMed] [Google Scholar]
- 23.Wei M, Li L, Meng R, et al. Suppressive effect of diazepam on IFN-gamma production by human T cells. Int Immunopharmacol. 2010;10(3):267–271. [DOI] [PubMed] [Google Scholar]
- 24.Maurer MJ, Ghesquieres H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson SE, Hsi ED. Epstein-Barr virus-positive B-cell lymphoma of the elderly at a United States tertiary medical center: an uncommon aggressive lymphoma with a nongerminal center B-cell phenotype. Hum Pathol. 2009;40(5):653–661. [DOI] [PubMed] [Google Scholar]
- 26.Ok CY, Li L, Xu-Monette ZY, et al. Prevalence and clinical implications of Epstein-Barr virus infection in de novo diffuse large B-cell lymphoma in Western countries. Clin Cancer Res. 2014;20(9):2338–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beltran BE, Castillo JJ, Morales D, et al. EBV-positive diffuse large B-cell lymphoma of the elderly: a case series from Peru. Am J Hematol. 2011;86(8):663–667. [DOI] [PubMed] [Google Scholar]
- 28.Sato A, Nakamura N, Kojima M, et al. Clinical outcome of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly in the rituximab era. Cancer Sci. 2014;105(9):1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn JS, Yang DH, Duk Choi Y, et al. Clinical outcome of elderly patients with Epstein-Barr virus positive diffuse large B-cell lymphoma treated with a combination of rituximab and CHOP chemotherapy. Am J Hematol. 2013;88(9):774779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.